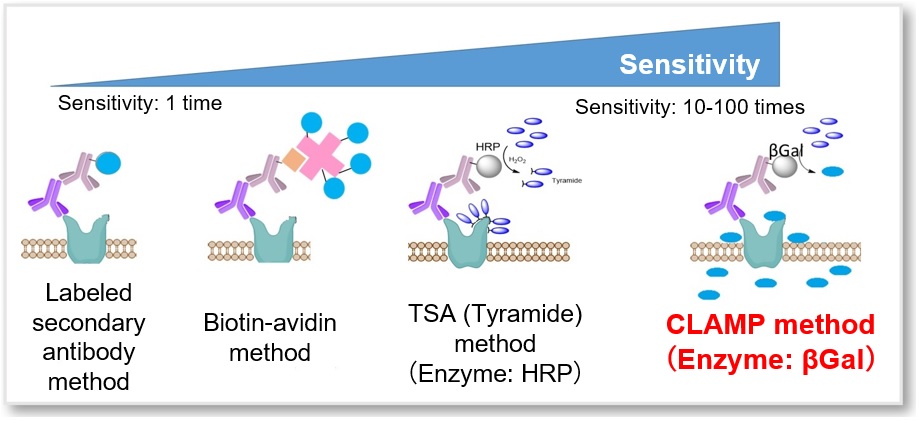
CLAMP F405-Signal Boosting

Substrate for Immunofluorescence
- Highly sensitive detection of cellular surface antigen expression
- High Selectivity and retention ability
-
Product codeC554 CLAMP F405-Signal Boosting
| Unit size | Price | Item Code |
|---|---|---|
| 10 µl | Find your distributors | C554-10 |
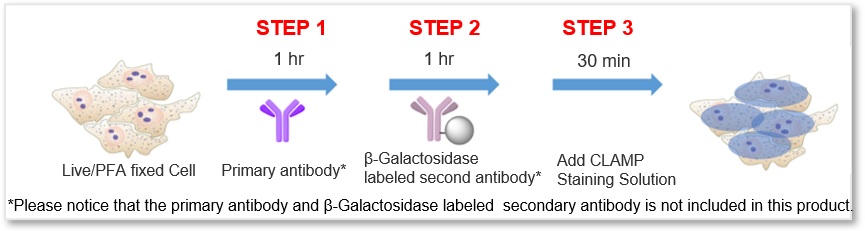
*Please notice that the primary antibody and β-Galactosidase labeled secondary antibody is not included in this product.
Description
Cell surface antigens that are specifically expressed in cancer and immune cells have been actively studied for early detection and treatment of cancer. However, many cell surface proteins have low expression levels, and most of them are difficult to detect and analyze by conventional techniques.
A fluorescence detection method using a fluorescence-labeled antibody is widely known as a specific detection method for cell surface proteins (fluorescence immunostaining method). However, it is difficult to apply this method for low expressed surface proteins due to low sensitivity. CLAMP F405-Signal Boosting allows you to detect these proteins.
*This product was developed with technical guidance and information provided by Prof. Yoshiki Katayama of Kyushu University.
Manual
Technical info
A highly sensitive CLAMP method (quinone methide-based catalyzed signal amplification) can be applied to live/fixed cells or tissue sections. In this method, using β-galactosidase-labeled secondary antibody and newly developed fluorescent dye CLAMP F405, the cells expressing a specific cell surface protein are selectively and highly sensitively stained. This fluorescent substrate, CLAMP F405, is actually non-fluorescent and non-permeable to the cell membrane in its beginning state. However, if the primary antibody binds to an antigen-presenting cell, the conjugated β-galactosidase will convert CLAMP F405 near the cell surface into a compound with a quinone methide structure. This compound is then permeable to the cell membrane; it will enter the cell and react with thiol and amino groups inside, forming covalent bonds and emitting fluorescence.
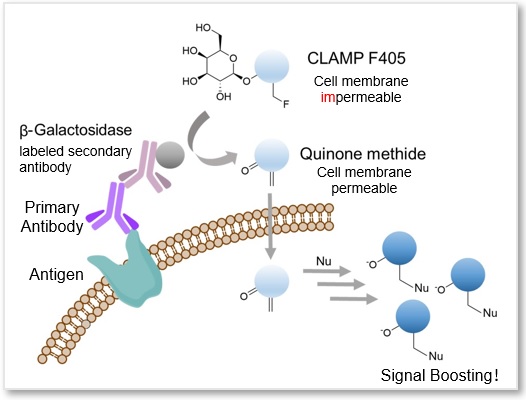
Reference: Noguchi, K. et al., “β-Galactosidase-Catalyzed Fluorescent Reporter Labeling of Living Cells for Sensitive Detection of Cell Surface Antigens”, Bioconjugate Chem., 2020, 31(7), 1740–1744.
Simple Procedure with High Sensitivity
The protocol is generally as simple as the secondary antibody method. After labeling the primary antibody, just add the β-galactosidase-labeled secondary antibody and the staining solution, then the sample is ready for detection.
Cell Line: HeLa Cells
Antigen: CD44
Instrument:
(Left) Fluorescent microscope Ex = 340 - 380 nm, Em = 435 - 485 nm
(Right) Flow Cytometer Ex = 405 nm, Em = 425 - 575 nm
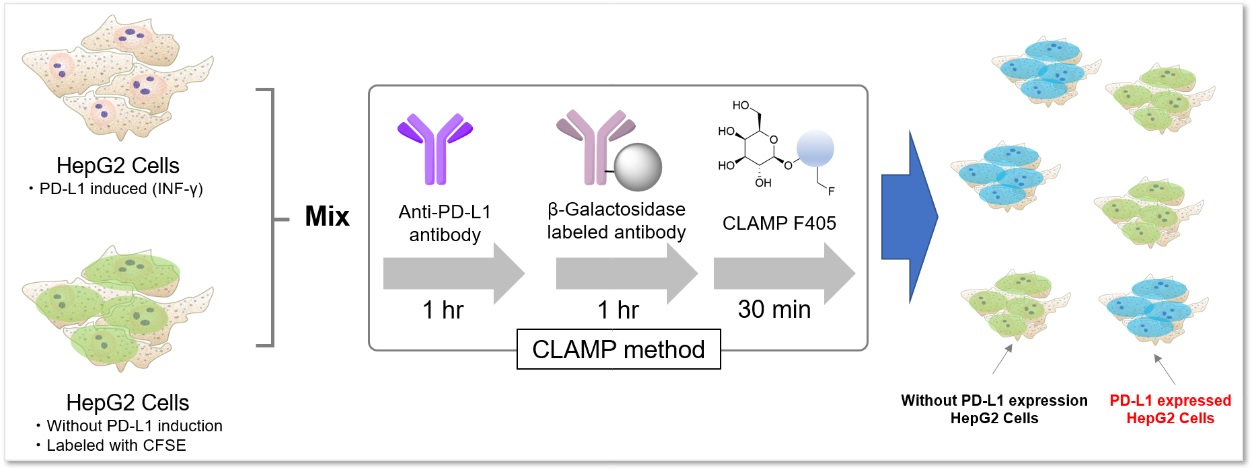
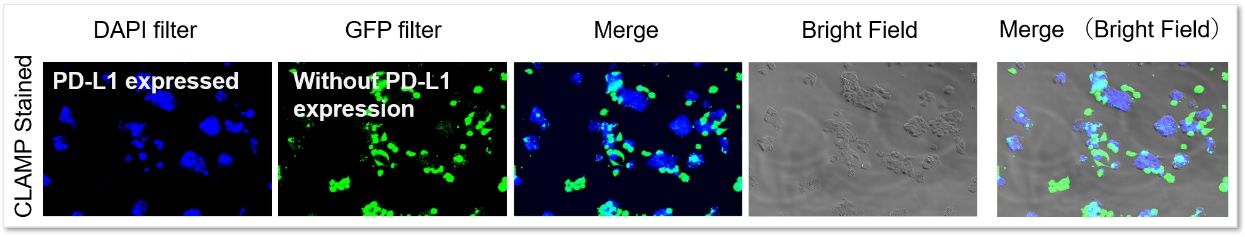
Application Data: PD-L1 Expressed HepG2 Cells
PD-L1 expressed HepG2 cells and CFSE-stained control cells were prepared and mixed. The results showed the CLAMP stained cells did not localize in the CFSE stained cells, indicating that the CLAMP method accurately differentiates PD-L1 expressed HepG2 cells, which was difficult using the secondary antibody method.
*CFSE: 5- or 6-(N-Succinimidyloxycarbonyl)fluorescein 3‘,6’-diacetate
<General Protocol>
・For adherent cells
<Experimental Conditions>
Blue (CLAMP): Ex=340-380 nm, Em=435-485 nm
Green (CFSE): Ex=450-490 nm, Em=500-550 nm
Even in situations where cells were adjacent to each other, only PD-L1-expressing cells were stained, and no misstaining of other cells was observed.
・Single Cell Analysis [SIEVEWELLTM (TOKYO OHKA KOGYO Co., LTD.)]

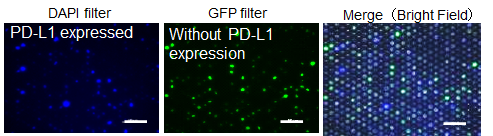 scale bar: 100 μm
scale bar: 100 μm
SIEVEWELLTM enables isolating cells into single cell and culturing on microwells. Combining the CLAMP staining method with SIEVEWELLTM, target cells can be separated accurately. After separation on the SIEVEWELLTM, picking rare cells and more precise cell separation are expected.
For details, please refer to the web page of TOKYO OHKA KOGYO Co., Ltd. https://www.sievewell.com/how-it-works
Application Data: FFPE Tissue Sections - Human Small Intestine
Using the CLAMP method and TSA (Tyramide signal amplification) method detected the αSMA (α-smooth muscle actin) and keratin on FFPE tissue sections of human small intestine samples. The results showed that the CLAMP method can easily co-stain with immunofluorescence. Please refer to the reference No.1 for a detailed protocol on the CLAMP method for tissue sections.
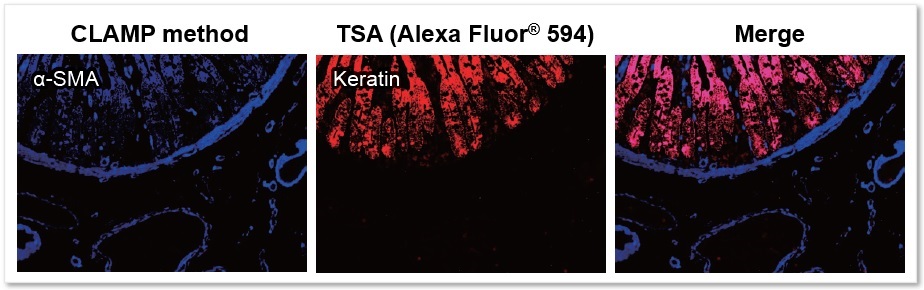
* The data was kindly provided by Dr. Masahiro Hirata, Department of Diagnostic Pathology, Kyoto University Hospital.
Application Data for each protocols in the manual
-
Protocol 1(Application Data: CD44 Antigen in HeLa Cells)
-
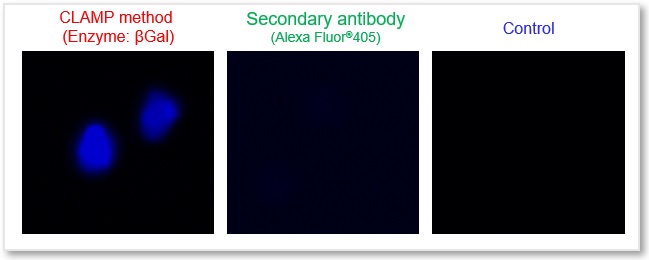
In accordance with protocol 1 of the manual, the CD44 antigen of live HeLa cells was detected using the CLAMP method. As a result, a clear difference in fluorescence intensity between the control and the stained samples was observed, indicating that the surface antigen can be detected with high sensitivity.

<Protocol>
1. Inoculated HeLa cells (1 x 105 cell/ml) into a 96-well microplate(100 μl/well) and incubated in a CO2 incubator at 37 ℃ overnight.
2. Discarded the supernatant and washed the cells with 100 μl of MEM (10% FBS) twice.
3. Added 100 μl of 2 μg/ml Anti-CD44 antibody (ab189524, abcam)/MEM (10% FBS) and incubated at 37℃ for 60 minutes.
4. Discarded the supernatant and washed the cells with 100 μl of MEM (10% FBS) twice.
5. Added 100 μl of x500 diluted β-Galactosidase-labeled Anti-Rabbit IgG/MEM (10% FBS) and incubated at 37℃ for 60 minutes.
6. Discarded the supernatant and washed the cells with 100 μl of HBSS twice.
7. Added 100 μl of Staining Solution and incubated at 37℃ for 30 minutes.
8. Discarded the supernatant and washed the cells with 100 μl of HBSS three times.
9. Observed the cells under a fluorescence microscope.(DAPI Filter: Ex = 320-360 nm, Em = 435-480 nm)
-
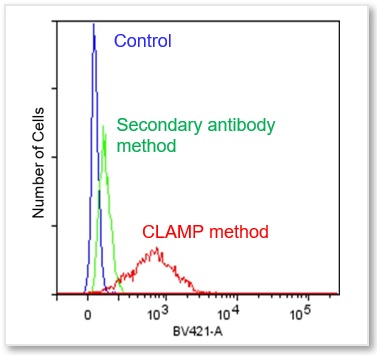
Protocol 2 (Application Data: CD 44 antigen in HeLa cells by Flow Cytometer)
-
In accordance with protocol 2 of the manual, the CD44 antigen of HeLa cells was measured using a flow cytometer. We compared the CLAMP method with the secondary antibody method. As a result, the CLAMP method can detect the antigen with higher sensitivity by a flow cytometer.
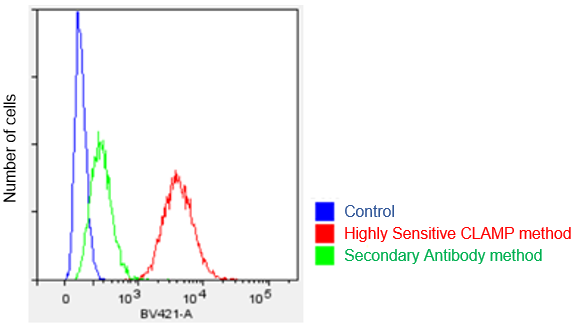
<Protocol>
1. Prepare 2 x 105 cells/tube of a cell suspension in a 1.5 ml microtube.
2. Centrifuge at 300 × g for 5 minutes and discard the supernatant.
3. Add 500 μl of 2 μg/ml Anti-CD44 antibody(ab189524, abcam)/MEM medium(10% FBS)and mix by pipetting.
4. Incubate at 37℃ for 60 minutes.
5. Perform the following washing step:
I. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
II. Add 500 μl of medium and mix by pipetting.
III. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
IV. Perform steps II and III again.
6. Add 500 μl of x500 β-Galactosidase labeled antibody (ab16774, abcam) /MEM medium (10% FBS), and mix by pipetting. (Secondary antibody method used Alexa Fluor405 antibody (ab175652, abcam))
7. Incubate at 37℃ for 60 minutes.
8. Perform the same washing step as in step 5 with HBSS. (Used HBSS instead of medium)
9. Add 500 μl of Staining Solution (Floating) and mix by pipetting.
10. Incubate at 37℃ for 30 minutes with stirring using a tube rotator. (RVM-101, Scinics)
11. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
12. Add 500 μl of HBSS and mix by pipetting.
13. Measure by a flow cytometer. (LSR Fortessa X-20、BD)
*BV421 filter set (Ex:405 nm, Em:430-470 nm)
-
Protocol 3 (Application Data: Anti-CD44 antigen in fixed HeLa Cells)
-
In accordance with protocol 3 of the manual, the CD44 antigen of fixed HeLa cells was detected using CLAMP method under live-cell experimental conditions. As a result, a clear difference in fluorescence intensity between the unstained sample and the fixed sample was observed, indicating that surface antigens can be detected with high sensitivity.

<Protocol>
Preparing PFA fixed cells
1. Inoculate HeLa cells (1 x 105 cell/ml) into a microplate and incubate in a CO2 incubator at 37 ℃ overnight.。
2. Discard the supernatant and wash the cells with 100 μl of PBS twice.
3. Add 100 μl of 4% PFA/PBS and incubate at room temperature for 30 minutes.
4. Add 100 μl of 0.1% Triton-X100/PBS and incubate at room temperature for 30 minutes.
5. Add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
Staining Protocol
1. Add 100 μl of 2 μg/ml Anti-CD44 antibody (ab189524, abcam) /Blocking Buffer and incubate at room temperature for 60 minutes.
2. Discard the supernatant and wash the cells with 100 μl of PBS twice.
3. Add 100 μl x500 β-Galactosidase labeled antibody (ab16774, abcam) /Blocking Buffer and incubate at room temperature
for 60 minutes.
4. Discard the supernatant and wash the cells with 100 μl of PBS twice.
5. Add 100 μl of the Staining Solution and incubate at 37℃ for 30 minutes.
6. Discard the supernatant and wash the cells with 100 μl of PBS three times.
7. Observe the cells under a fluorescence microscope.(DAPI filter: Ex = 320-360 nm, Em = 435-480 nm)
-
Protocol 4 (Application Data: PD-1 antigen in fixed MOLT4 Cells)
-
In accordance with protocol 2 of the manual, the PD1 antigen in fixed MOLT4 cells was detected using a flow cytometer by CLAMP method and secondary antibody method. The results showed that the CLAMP method had a higher sensitivity than the secondary antibody method with flow cytometer.
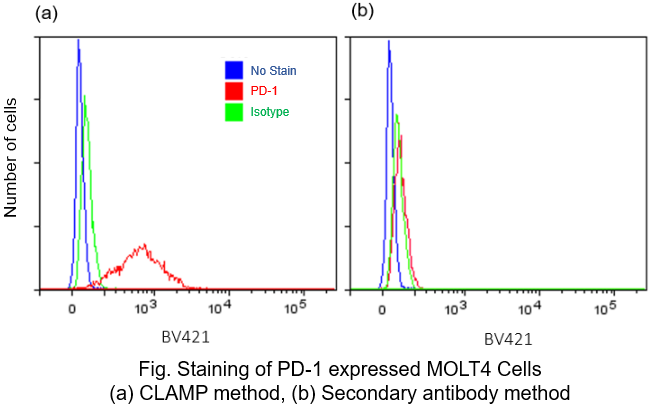
<Protocol>
Preparing fixed cells
1. Prepared MOLT4 cells (2 x 105 cells/tube) of a cell suspension in a 1.5 ml microtube.
2. Centrifuged at 300 × g for 5 minutes and discarded the supernatant.
3. Added 500 μl of 4% PFA/PBS and mixed by pipetting.
4. Incubated at room temperature for 30 minutes, then centrifuged at 300 × g for 5 minutes.
5. Discarded the supernatant, added 500 μl of 0.1% Triton-X100/PBS, and mixed by pipetting.
6. Incubated at room temperature for 30 minutes, then centrifuged at 300 x g for 5 minutes.
7. Discarded the supernatant, added 500 μl of Blocking Buffer (10% Blocking One/PBS), and mixed by pipetting
8. Incubated at room temperature for 30 minutes, then centrifuged at 300 x g for 5 minutes, Used the fixed cells for the staining process.
Staining protocol
1. Add 500 μl of 1 μg/ml mouse Anti-PD1 antibody(ab52587, abcam)/Blocking Buffer and mix by pipetting.
2. Incubate at room temperature for 60 minutes.
3. Perform the following washing step:
I. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
II. Add 500 μl of Blocking Buffer and mix by pipetting.
III. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
IV. Perform steps II and III again.
4. Add 500 μl of X3,000 β-Galactosidase-labeled Anti-mouse antibody(ab136775, abcam) /Blocking Solution and mix by pipetting.
5. Incubate at room temperature for 60 minutes.
6. Perform the same washing step as in step 3.
7. Add 500 μl of Staining Solution (Floating ) and mix by pipetting。
8. Incubate at 37℃ for 30 minutes with stirring using a tube rotator.
9. Centrifuge at 300 x g for 5 minutes and discard the supernatant.
10. Add 500 μl of Blocking Buffer and mix by pipetting.
11. Measure by a flow cytometer.(LSR Fortessa X-20, BD)
*BV421 filter set (Ex:405 nm, Em:430-470 nm)
-
Protocol 5 (Application Data: Actin in frozen tissue section of mouse liver)
-
In accordance with protocol 5 of the manual, the CLAMP method without primary antibody was compared with the normal CLAMP method. As a result, the CLAMP method was found to be highly sensitive and selective for Actin staining.
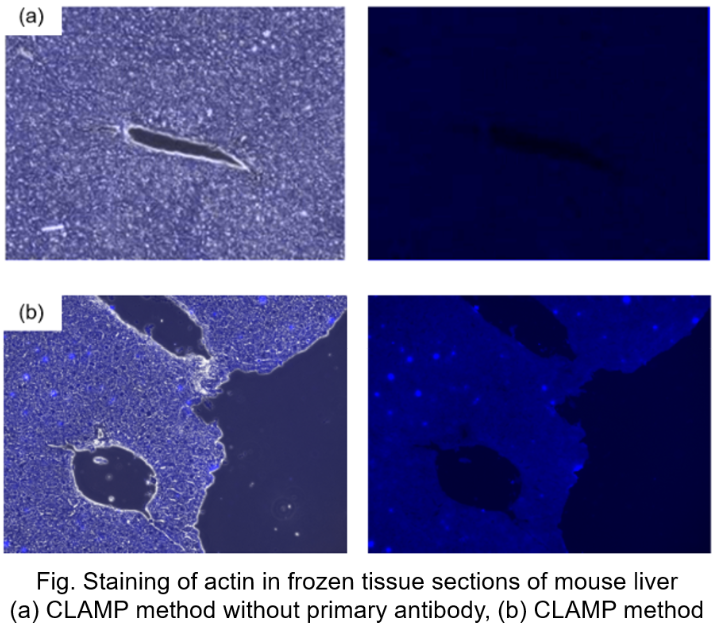
<Protocol>
PFA Fixation
1. Bring a frozen tissue section on a slide glass to room temperature.
2. Draw a border around the tissue section using a water-repellent pen.
3. Add 100 μl of 4% PFA/PBS and incubate at room temperature for 30 minutes.
4. Discard the supernatant, add 100 μl of 0.1% Triton-X100/PBS and incubate at room temperature for 30 minutes.
5. Discard the supernatant, add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
Staining Protocol
1. Discard the supernatant, add 100 μl of 2 μg/ml Anti-actin antibody (GTX109639, GeneTex)/Blocking Buffer and incubate at room temperature for 60 minutes.
2. Discard the supernatant and wash with Blocking Buffer twice.
3. Add 100 μl of x500 β-Galactosidase labeled Antibody (ab16774, abcam)/Blocking Buffer and incubate at room temperature for 60 minutes.。
4. Discard the supernatant and wash with Blocking Buffer twice.
5. Add 100 μl of the Staining Solution and incubate at 37℃ for 30 minutes.
6. Discard the supernatant and wash with PBS three times.
7. Observe the cells under a fluorescence microscope.(DAPI filter: Ex = 320-360 nm, Em = 435-480 nm)
-
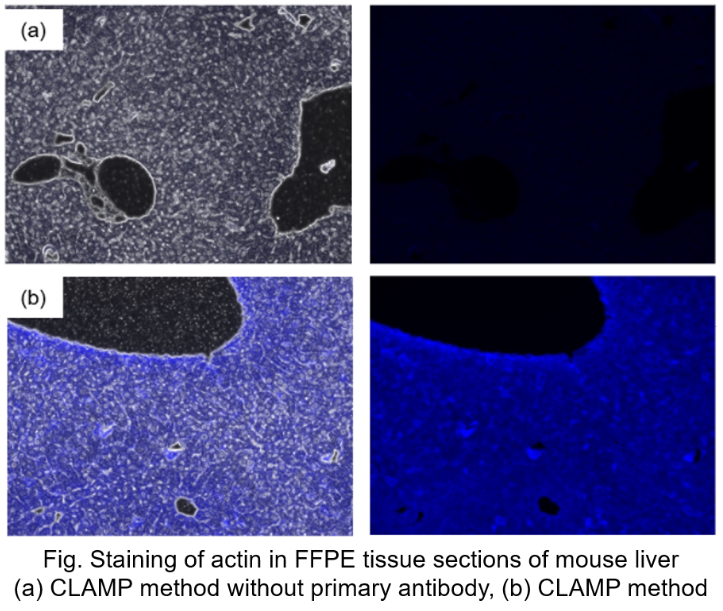
Protocol 6 (Application Data: Actin in FFPE tissue sections of mouse liver)
-
In accordance with protocol 6 of the manual, Actin in mouse liver FFPE tissue sections was measured using fluorescence microscopy. The CLAMP method without primary antibody was compared with the conventional CLAMP method. As a result, the CLAMP method was found to be highly sensitive and selective for Actin staining.
<Protocol>
Antigen Activation
1. Prepare FFPE tissue section on a slide glass.
2. Incubate at 60℃ for 60 minutes.
3. Incubate at room temperature for 15 minutes.
Note: Use a humidity chamber not dry the sections during the following processes.
4. Soak the slide with lemosol for 5 minutes. Repeat this step.
5. Soak the slide with ethanol for 5 minutes.
6. Soak the slide with 90% ethanol aqueous solution for 5 minutes.
7. Soak the silde with 80% ethanol aqueous solution for 5 minutes.
8. Wash the slide by soaking in ultrapure water, followed by PBS.
9. Soak the slide with antigen activation solution (pH=9), and heated to 95℃ for 40 minutes.
10. Incubate at room temperature for 60 minutes.。
11. Wash the slide by soaking in ultrapure water, followed by PBS.
Staining protocol
1. Draw a border around the tissue section using a water-repellent pen.
2. Add 100 μl of Blocking Buffer and incubate at room temperature for 30 minutes.
3. Discard the supernatant, add 100 μl of 2 μg/ml Anti-actin antibody (GTX109639, GeneTex) /Blocking Buffer and incubate at room temperature for 60 minutes.
4. Discard the supernatant, add 100 μl of PBS and incubate at room temperature for 3 minutes. Repeat this step twice.
5. Add 100 μl of x500 β-Galactosidase labeled secondary antibody (ab16774, abcam社) /Blocking Buffer and incubate at room temperature for 60 minutes.
6. Discard the supernatant, add 100 μl of PBS and incubate at room temperature for 3 minutes. Repeat this step twice.
7. Add 100 μl of Staining Solution and incubate at 37℃ for 30 minutes.
8. Discard the supernatant, add 100 μl of PBS and incubate at room temperature for 3 minutes. Repeat this step twice.
9. Observe the cells under a fluorescence microscope.
References
| No. | Sample | Reference (Link) |
|---|---|---|
| 1 | Tissue (Human tonsil and duodenum) |
Hirata, M., et al. "Galactosidase-catalyzed fluorescence amplification method (GAFAM): sensitive fluorescent immunohistochemistry using novel fluorogenic β-galactosidase substrates and its application in multiplex immunostaining." Histochem Cell Biol (2022). DOI:10.1007/s00418-022-02162-5. |
Q & A
-
Q
What cell types and antigens have been stained successfully by this kit?
-
A
Please refer to the following table.
Sample Type Sample in details Antigen Instrument Cells HeLa cells CD44, GLUT1 Microscope, FCM Cells HeLa cells CD63, CD9 Microscope Cells A549 cells CD44 Microscope Cells HepG2 cells PD-L1 Microscope Cells HL60 cells CD44, CD33 Microscope, FCM Cells MOLT4 cells PD-1, CD44 FCM Cells T2 cells Antibody FCM Cells myocyte cells Antibody Microscope Cells bjab cells class I Antigen Microscope Cells erythrocyte Surface Antigen FCM Cells Macrophage induced
THP-1 cellsABCA 1 Microscope Tissue FFPE tissue section
(Mouse, Liver)Actin Microscope Tissue FFPE tissue section
(Human, Kidney)Actin Microscope Tissue FFPE tissue section
(Human, Big intestine, Lung)p16 Microscope Tissue FFPE tissue section
(Human, Small intestine)CD45,α-SMA Microscope Tissue Frozen tissue section
(Mouse, Brain)Iba1 Microscope
-
Q
Can I store the stained sample for a long period of time?
-
A
For live cells, after staining and PFA fixation, the sample in PBS solution can be stored at -20℃ for 1 week. For fixed samples, can also be stored at -20℃ for 1 week.
-
Q
Is endogenous β-Galactosidase affect the result?
-
A
In the case of living cells, CLAMP F405 does not react with endogenous βGalactosidase because it is not membrane permeable. Also, in the case of fixed cells and tissues, there is no background fluorescence increase in PBS solution.
-
Q
Where can I get the β-Gal labeled secondary antibodies?
-
A
We do not have the primary antibodies or β-galactosidase labeled antibodies. The following is a list of antibodies that we have tested successfully.
Origin
Company
Catalog#
human
arigo biolaboratories
ARG21670
mouse
arigo biolaboratories
ARG21521
Human
arigo biolaboratories
ARG23882
human
arigo biolaboratories
ARG24060
rat
arigo biolaboratories
ARG21691
mouse
abcam
ab136775
rat
abcam
ab136716
goat
abcam
ab136712
rabbit
abcam
ab136774
mouse
abcam
ab130781
mouse
southernbiotech
1010-06
mouse
southernbiotech
1020-06
mouse
southernbiotech
1030-06
human
southernbiotech
2010-06
human
southernbiotech
2040-06
human
southernbiotech
2060-06
rat
southernbiotech
3030-06
rabbit
southernbiotech
4010-06
mouse
Sigma Aldrich
401607
-
Q
Please tell me the recommended channel or filter for each individual instrument.
-
A
Fluorescent microscope: DAPI filter, Ex = 320-380 nm; Em = 435-485 nm
Confocal microscope: Ex = 405 nm, Em = 425-475 nm
Flow Cytometer: Ex = 405nm, Em: BV421, Pacific Blue (450/50 nm)
-
Q
Is the CLAMP F405-Signal Boosting DMSO solution stable after repeated freezing and thawing?
-
A
We have confirmed that the DMSO solution is still stable after 20 times of freezing and thawing.
-
Q
Can I store the staining solution for a long period of time?
-
A
No, the staining solution should be used on the day be prepared.
-
Q
If the background fluorescence is high, is there any suggestions?
-
A
Please follow the [Considering proper antibody concentrations] steps in the manual to find out the proper concentration. For floating cells, please use a tube rotator during the staining step.
-
Q
If the fluorescent intensity is really low, what should I do?
-
A
Prepare a new Staining Solution and stain for 30-60 minutes at 37°C. If the fluorescence intensity is still weak, the possible cause is the primary/secondary antibody concentration may not be appropriate. Please follow the [Considering proper antibody concentrations] steps in the manual to find out the proper concentration.
Handling and storage condition
| 1. Hazardous material Class 4, Petroleum No. 3, Hazard class III, 2. Fire strictly prohibited 3. Storage method: Frozen | |
|
Danger / harmful symbol mark |

|
|---|---|













