Fluo 3-AM

Reagent for Monitoring Intracellular Calcium Ion
-
Product codeF023 Fluo 3-AM
-
CAS No.121714-22-5
-
Chemical name1-[2-Amino-5-(2,7-dichloro-6-acetoxymethoxy-3-oxo-9-xanthenyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid,tetra(acetoxymethyl)ester
-
MWC51H50Cl2N2O23=1129.85
| Unit size | -- | |
|---|---|---|
| 1 mg | Please inquire distributors about price. | |
Description
Product Description
Fluo 3 is a long wavelength calcium probe that is practically nonfluorescent in its free ligand form, but its fluorescence increases 60-80 times when it forms complexes with calcium. Thus, it has been widely used with confocal laser fluorescent microscopy because the microscope has an argon laser. The long wavelength of the fluorescent signal is also convenient for minimizing photodamage to sample cells. Fluo 3 is also useful for caged calcium and others that are cleaved by the photoirradiation in the UV region. Fluo 3-AM is an acetoxymethyl ester derivative of Fluo 3 that can be easily loaded into cells by incubation.
Hydrolysis of AM ester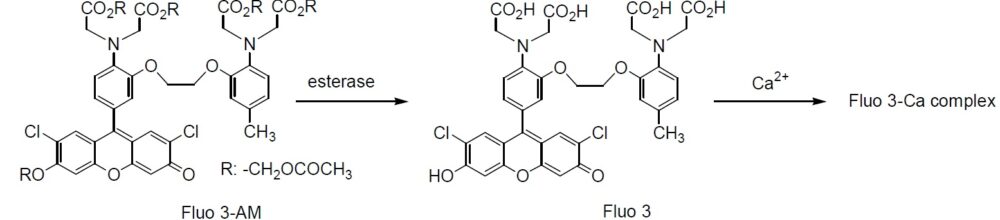
Technical info
General Protocol (for Human T cells)*
Reagents:
・2 mM Fluo 3-AM/DMSO (1 mg Fluo 3-AM in 442 μl DMSO)
・Pluronic F127
・Hanks Ebalanced salt solution (HBSS)
・HEPES buffer saline (10 mM HEPES, 1 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM glucose, 0.1% BSA, pH 7.4)
Protocol:
1. Add 16.5 mg Pluronic F127 to Fluo 3-AM/DMSO solution. Pluronic F127 prevents aggregation of Fluo 3-AM in HBSS and helps uptake with cells.
2. Dilute the Fluo 3-AM solution with HBSS to prepare 4 μM Fluo 3-AM working solution.
3. Add the Fluo 3-AM working solution to the cells and incubate at 37oC for 20 min.
4. Add HBSS containing 1% fetal Calf serum equivalent to 5 times the volume of Fluo 3 -AM working solution (step 3).
5. Wash the cells 3 times with HEPES buffer saline. Then resuspend the cells to prepare 1×105 cells per ml solution using HEPES buffer saline.
6. Incubate at 37oC for 10 min. Then use the cells for fluorescent calcium ion detection.
7. Monitor the fluorescence at 528 nm (excitation: 490-500 nm).
*Cell staining conditions depend on cell types, so it is necessary to optimize the conditions for each experiment.
References
References
1. A. Minta, et al., Fluorescent Indicators for Cytosolic Calcium Based on Rhodamine and Fluorescein Chromophores. J Biol Chem. 1989;264:8171-8178.
2. J. P. Kao, et al., Photochemically Generated Cytosolic Calcium Pulses and Their Detection by Fluo-3. J Biol Chem. 1989;264:8179-8184.
3. M. Eberhard, et al., Kinetics of Calcium Binding to Fluo-3 by Stopped-Flow Fluorescence. Biochem Biophys Res Commun. 1989;163:309-314.
4. A. Hernandez-Cruz, et al., Subcellular Calcium Transients Visualized by Confocal Microscopy in a Voltage-clamped Vertebrate Neuron. Science. 1990;247:858-862.
5. A. H. Cornell-Bell, et al., Glutamate Induces Calcium Waves in Cultured Astrocytes: Long-Range Glial Signaling. Science. 1990;247:470-473.
6. D. A. Williams, Quantitative Intracellular Calcium Imaging with Laser-scanning Confocal Microscopy. Cell Calcium. 1990;11:589-597.
7. D. A. Williams, et al., Confocal Imaging of Ionised Calcium in Living Plant Cells. Cell Calcium. 1990;11:291-297.
8. P. A. Vandenberghe, et al., Flow Cytometric Measurement of Cytoplasmic Free Calcium in Human Peripheral Blood T Lymphocytes with Fluo-3, A New Fluorescent Calcium Indicator. J Immunol Methods. 1990;127:197-205.
9. M. Iino, et al., Visualization of Neural Control of Intracellular Ca2+ Concentration in Single Vascular Smooth Muscle Cells in situ. EMBO J. 1994;13:5026-5031.
10. M. E. Dailey, et al., Spontaneous Ca2+ Transients in Developing Hippocampal Pyramidal Cells. J Neurobiol. 1994;25:243-251.
11. M. Burnier, et al., Confocal Microscopy to Analyze Cytosolic and Nuclear Calcium in Cultured Vascular Cells. Am J Physiol. 1994;266:C1118-C1127.
12. E. Donnadieu, et al., Ca2+ Signaling in Endothelial Cells Stimulated by Bradykinin: Ca2+ Measurement in the Mitochondria and the Cytosol by Confocal Microscopy. Cell Calcium. 1996;20:53-61.
13. M. Ikeda, et al., Separate Analysis of Nuclear and Cytosolic Ca2+ Concentrations in Human Umbilical Vein Endothelial Cells. J Cell Biochem. 1996;63:23-36.
14. J. E. Merritt, et al., Use of fluo-3 to Measure Cytosolic Ca2+ in Platelets and Neutrophils Loading Cells with the Dye, Calibration of Traces, Measurements in the Presence of Plasma, and Buffering of Cytosolic Ca2+. Biochem J. 1990;269:513-519.
Handling and storage condition
| Appearance: | Red powder or solid |
|---|---|
| Purity (HPLC): | ≧ 85.0 % |
| Solubility in acetonitrile: | To pass test (clear, orange) |
| Fluorescence spectrum: | To pass test |
| NMR spectrum: | Authentic |
| -20°C, Protect from light |








