Peroxidase Labeling Kit - NH2 (for 1mg)

Antibody / Protein Labeling
- Only 3 hours to get conjugates
- All processes in a single Filtration tube
- High recovery of conjugates
-
Product codeLK51 Peroxidase Labeling Kit - NH2 (for 1mg)
| Unit size | -- | |
|---|---|---|
| 1 sample | Please inquire distributors about price. | |
| 1 sample | ・NH2-Reactive Peroxidase ・Washing Buffer ・Reaction Buffer ・Storage Buffer ・Filtration Tube ・15 ml Tube (for counterbalance) |
1 tube 10 ml x1 1.2 ml x1 10 ml x1 1 tube 1 tube |
|---|
Description
Product Description
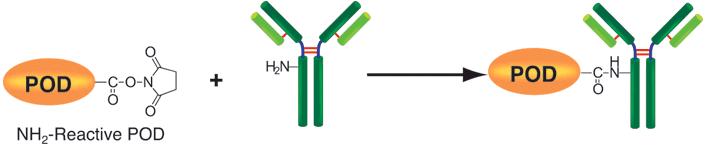
Peroxidase Labeling Kit-NH2 is used mainly for the preparation of peroxidase-labeled IgG for enzyme immunoassay (EIA) and for the preparation of peroxidase-labeled antigen for competitive EIA. NH2-reactive peroxidase, a component of this kit, has succinimidyl groups (NHS) and reacts with proteins or other molecules that have an amino group in their structures (Fig. 1). This kit contains all the reagents necessary for the labeling process, including storage buffer. The labeling process is simple: mix IgG with NH2-reactive peroxidase and incubate at 37ºC for 2 hours. The NH2-reactive peroxidase forms a covalent link with the target molecule without any activation process. The distance of the NHS from peroxidase is about 1.2 nm, half of the radius of the peroxidase molecule. Therefore, when the peroxidase-labeled IgG is used for EIA, the labeling efficiency of the NH2-reactive peroxidase is high enough to eliminate the purification process after labeling. Also, peroxidase labeling will not affect the affinity of the target molecule. If a high-purity conjugate is required after labeling, simply use an affinity column or a gel-permeation column. When labeling small molecules, excess molecules can be removed by using the filtration tubes included in this kit. Because the amino groups of NH2-reactive peroxidase are blocked, no self-conjugation is possible.
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info
♦ The molecular weight of the protein to be labeled with this kit should be greater than 50,000.
♦ The molecular weight of the small amine compound to be labeled with this kit should be smaller than 5,000.
♦ IgG or peroxidase-conjugated IgG is always on the membrane of the filtration tube during the labeling process.
♦ If the IgG solution contains other proteins with a molecular weight greater than 10,000, such as BSA or gelatin, purify the IgG solution before labeling peroxidase with this kit.
♦ If the IgG solution contains small insoluble materials,centrifuge the solution and use the supernatant for labeling.
Sandwich ELISA
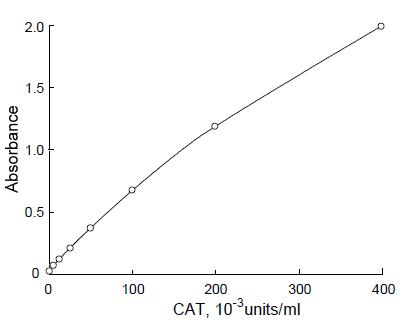
Fig. 2 Sandwich ELISA of CAT (chloramphenicol acetyl transferase) assay.
Plate: 2 μg/ml anti-CAT antibody (rabbit anti sera)-coated high binding plate
CAT: 0-400 x 10-3units/ml PBST
Peroxidase-conjugated anti-CAT antibody: Prepared by Peroxidase Labeling Kit-NH2.1μg/ml PBST+blocking reagent
Substrate: TMB peroxidase substrate
Western blot
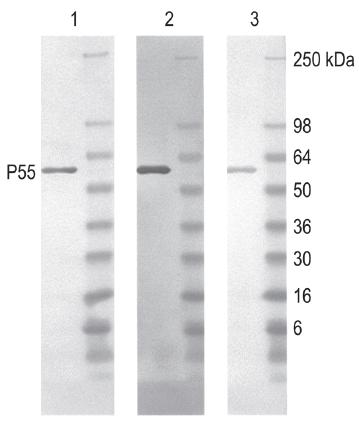
Fig. 3 Western blot using peroxidase-labeled monoclonal antibody to SIV p24 Gag(2F12).
SIV P55 and molecular weight markers were analyzed in blot 1, 2, and 3.
Blot 1: conjugate prepared using Peroxidase Labeling Kit-NH2
Blot 2: conjugate prepared using Peroxidase Labeling Kit-SH
Blot 3: primary antibody and peroxidase-conjugated secondary antibody (commercially available).
The western blotting using peroxidase-labeled primary antibody gives abetter result than using peroxidase-labeled secondary antibody. In most cases, the sensitivity of the conjugate prepared with Peroxidase/ Alkaline phosphatase Labeling Kit-SH is higher than Labeling Kit-NH2 due to the site specific conjugation on the antibody.
References
1. A. Miyagawa-Yamaguchi, N. Kotani, and K. Honke, “Expressed Glycosylphosphatidylinositol-Anchored Horseradish Peroxidase Identifies Co-Clustering Molecules in Individual Lipid Raft Domains”, PLoS ONE., 2014, 9, (3), e93054.
2. J. Zhang, D. Klufas, K. Manalo, K. Adjepong, J.O. Davidson, G. Wassink, L. Bennet, A.J. Gunn, E.G. Stopa, K. Liu, M. Nishibori, and B.S. Stonestreet, “HMGB1 Translocation After Ischemia in the Ovine Fetal Brain”, J. Neuropathol. Exp. Neurol.., 2016, 75, (6), 527.
3. M. Okumura, T. Ozawa, H. Hamana, Y. Norimatsu, R. Tsuda, E. Kobayashi, K. Shinoda, H. Taki, K. Tobe, J. Imura, E. Sugiyama, H. Kishi, and A. Muraguchi, “Autoantibodies reactive to PEP08 are clinically related with morbidity and severity of interstitial lung disease in connective tissue diseases”, Eur. J. Immunol.., 2018, 48, (10), 1717.
4. R. Sugisawa, G. Komatsu, E. Hiramoto, N. Takeda, K. Yamamura, S. Arai, and T. Miyazaki, “Independent modes of disease repair by AIM protein distinguished in AIM-felinized mice”, Sci. Rep.., 2018, 8, 13157.
5. S. Takatsuka, T. Inukai, S. Kawakubo, T. Umeyama, M. Abe, K. Ueno, Y. Hoshino, Y. Kinjo, Y. Miyazaki, and S. Yamagoe, “Identification of a Novel Variant Form of Aspergillus fumigatus CalC and Generation of Anti-CalC Monoclonal Antibodies”, Med Mycol J., 2019, 60, (1), 11.
6. T. Sasaki, K. Liu,T. Agari, T. Yasuhara, J. Morimoto, M. Okazaki, H. Takeuchi, A. Toyoshima, S. Sasada, A. Shinko, A. Kondo, M. Kameda, I. Miyazaki, M. Asanuma, CV. Borlongan, M. Nishibori, and I. Date, “Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease”, Exp. Neurol.., 2016, 275, 220.
7. T. Tsumuraya, I. Fujii, M. Inoue, A. Tatami, K. Miyazaki, and M. Hirama, “Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C”, Toxicon., 2006, 48, (3), 287.
8. W. Jin, K. Yamada, M. Ikami, N. Kaji, M. Tokeshi, Y. Atsumi, M. Mizutani, A. Murai, A. Okamoto, T. Namikaw, Y. Baba, and M. Ohta, “Application of IgY to sandwich enzyme-linked immunosorbent assays, lateral flow devices, and immunopillar chips for detecting staphylococcal enterotoxins in milk and dairy products”, J. Microbiol. Methods., 2013, 92, (3), 323.
9. W.W.P.N. Weerakoon, M. Sakase, N. Kawate, M.A. Hannan, N. Kohama, and H. Tamada, “Plasma IGF-I, INSL3, testosterone, inhibin concentrations and scrotal circumferences surrounding puberty in Japanese Black beef bulls with normal and abnormal semen”, Theriogenology., 2018, 114, (1), 54.
10. Y. Watanabe, Y. Kazuki, K. Kazuki, M. Ebiki, M. Nakanishi, K. Nakamura, M. Yoshida Yamakawa,H. Hosokawa, T. Ohbayashi, M. Oshimura, and K. Nakashima, “Use of a Human Artificial Chromosome for Delivering Trophic Factors in a Rodent Model of Amyotrophic Lateral Sclerosis”, Mol Ther Nucleic Acids., 2015, 4, (10), e253.
11. Y.S. Kim, D.H. Jung, I.S. Lee, B.J. Pyun and J.S. Kim, “Osteomeles schwerinae extracts inhibits the binding to receptors of advanced glycation end products and TGF-β1 expression in mesangial cells under diabetic conditions”, Phytomedicine., 2016, 23, (4), 388.
Q & A
-
Q
Can I use this Kit for Fab or Fab E labeling?
-
A
Yes, you can label Fab or Fab E using this kit. The recovery of the conjugate should be over 80%.
-
Q
Can I use this Kit for other proteins?
-
A
Yes, if the molecular weight is greater than 50,000 or less than 5,000 and it has a reactive primary or secondary amino group. If the molecular weight is higher than 50,000, follow the labeling protocol for IgG and use 0.5-1 nmol of sample protein for LK11-10.
If the molecular weight is less than 5,000, follow the labeling protocol for small molecules. If the molecular weight is higher than 5,000 but lower than 50,000, contact our customer service at info@dojindo.com or 1-877-987-2667 for more information.
-
Q
Can I use this Kot to label an ologonucleotide or oligopeptide?
-
A
Yes, if the molecular weight is less than 5,000 and it has a reactive primary or secondary amino group. Follow the labeling protocol for small molecules.
-
Q
What is the minimum amount of IgG that can be labeled whith LK11-10?
-
A
The minimum amount is 50 μg. There is no significant difference in sensitivity and background between 50 μg and 200 μg of IgG.
Though 10 μg IgG can still be labeled using this kit, the background will be higher.
-
Q
How many peroxidase moleculas per IgG are introduced?
-
A
The average number of peroxidase molecule per IgG is 1 to 3.
-
Q
Does unconjugated NH2-reactive peroxidase still have an activated ester after the labeling reaction to IgG?
-
A
No. It is completely hydrolyzed during the reaction.
-
Q
Does NH2-reactive peroxidase form an oligomer during the labeling reaction?
-
A
No. Since all amino groups of NH2-reactive peroxidase are blocked, no oligomerization is possible.
-
Q
Do I have to use Storage buffer included whith the kit?
-
A
No, you do not have to use Storage buffer from the kit. You can choose any kind of buffer appropriate for your experiment.
However, the Storage buffer helps to increase the stability of the peroxidase conjugate.
-
Q
Does Storage buffer contain animal roducts or polymers?
-
A
No, Storage buffer does not contain any animal products, polymers, or heavy metal ions.
Handling and storage condition
| 0-5°C, Protect from moisture |











