-SulfoBiotics- HSip-1

Biosulfur Analysis
-
Product codeSB21 -SulfoBiotics- HSip-1
-
CAS No.1346220-52-7(free base)
-
Chemical nameN-[3',6'-Dihydroxy-3-oxo-3H-spiro(isobenzofuraN-1,9'-xanthen)-5-yl]-(1,4,7,10-tetraazacyclododecane-1-acetamide-κN1, κN4, κN7, κN10) copper(2+) bis(trifluoroaceta
-
MWC34H33CuF6N5O10=849.19
| Unit size | Price | Item Code |
|---|---|---|
| 1 mg | Find your distributors | SB21-10 |
Description
It has been recognized that hydrogen sulfide (H2S) has an important role as a physiological active substance for vasodilation, cytoprotection, and modulation of insulin secretion. H2S is considered as a gaseous molecule such as nitric oxide and carbon monoxide. However, around 80% of the total sulfide exists as hydrogen sulfide anion (HS- ) under physiological condition, since the pKa is about 7. In addition, HS- easily converts to various biochemical molecules such as persulfides and polysulfides, which react with sulfhydryl moieties in a living body. -SulfoBiotics- HSip-1 is a novel fluorescent probe to detect H2S selectively and it emits strong green fluorescence when it reacts with H2S. -SulfoBiotics-HSip-1 DA is cell membrane permeable and it enables fluorescent imaging of intracellular H2S.
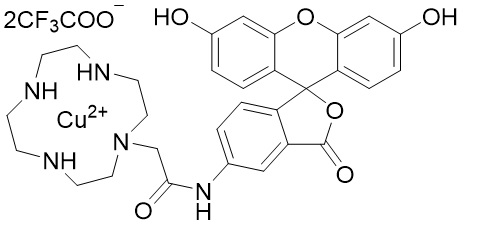
Fig. 1 Chemical structure of HSip-1
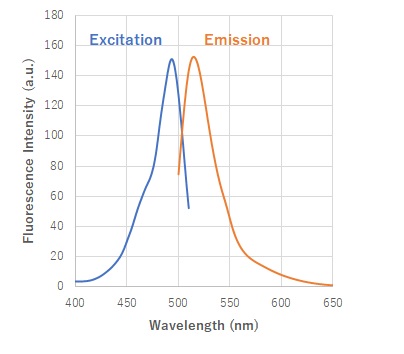
Fig. 2 Excitation and emission spectra of HSip-1 reacted with H2S
(Em: 491nm/Ex: 516nm)
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info
1. Preparation of Standard Curve
To quantitate the amount of hydrogen sulfide in a sample, A standard curve was derived from the serial dilutions of hydrogen sulfide donor, Sodium Sulfide.
1) HSip-1 stock solution (10 mmol/l) was diluted with PBS to prepare 200 μmol/l HSip-1 working solution.
2) Sodium Sulfide (-SulfoBiotics- Sodium Sulfide (Na2S), 7.8 mg) were dissolved in 1 ml of de-oxygenated H2O prepared by bubbling of nitrogen gas (100 mmol/l Na2S solution).
3) Na2S solution (100 mmol/l, 20 μl) was added to 980 μl of de-oxygenated H2O to prepare 2 mmol/l Na2S solution.
4) Na2S solution (2 mmol/l,100 μl) was added to 900 μl of de-oxygenated H2O to prepare 200 μmol/l Na2S solution.
5) Na2S solution (200 μmol/l) was diluted with de-oxygenated H2O to prepare various concentration of Na2S solution by serial dilution (200, 100, 50, 25, 12.5, 6.3, 3.2, 0 μmol/l).
6) HSip-1 working solution (200 μmol/l, 350 μl) was added to 300 μl of the Na2S solutions and mixed using a vortex mixer.
7) The solutions were incubated at room temperature for 30 minutes and 200 μl of the solution were transfered to each well (96-well plate).
8) The fluorescence intensities were measured at 516 nm (λex=491 nm) with a microplate reader.
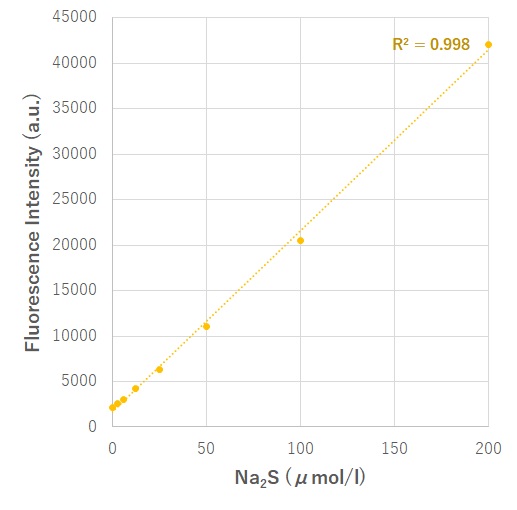
Fig. Fluorescence intensity changes at 516 nm with various concentrations of hydrogen sulfide.
2. Experimental Example with HeLa Cells
1) Inoculate HeLa cells in 96-well microplate and incubate the cells in a CO2 incubator overnight.
2) Wash the cells with PBS buffer and remove the supernatant.
3) Add Lysis buffer * 100 μl to a well and pipet it to lyse cells.
4) Add HSip-1 working solution 100 μl to a well and incubate the cells at room temperature for 30 minutes.
5) Measure the fluorescence intensity on a plate reader.( Ex: 491 nm, Em: 516 nm)
* Lysis buffer: 6 mol/l Urea, 2% SDS, 150 mmol/l Tris-HCl (pH7.4)
The hydrogen sulfide concentration was 3 to 9 μmol/l in HeLa cells. The concentration was obtained from the standard curve, according to the fluorescence intensity of the sample.
References
1) K.Sasakura, K.Hanaoka, N.Shibuya, Y.Mikami, Y. Kimura, T.Komatsu, T.Ueno, T.Terai, H.Kimura, and Tetsuo Nagano, "Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide", J. Am. Chem. Soc., 2011, 133, (45), 18003–18005.
2) H.Kimura, N.Shibuya, and Y.Kimura, "Hydrogen Sulfide Is a Signaling Molecule and a Cytoprotectant", Antioxid Redox Signal., 2012, 17, (1), 45-47.
3) H.Jurkowska, Heather B. Roman, Lawrence L. Hirschberger, K.Sasakura, T.Nagano, K.Hanaoka, J.Krijt, and Martha H. Stipanuk, "Primary hepatocytes from mice lacking cysteine dioxygenase show increased cysteine concentrations and higher rates of metabolism of cysteine to hydrogen sulfide and thiosulfate", Amino Acids., 2014, 48, (5), 1353-1365.
4) E.Marutani, M. Sakaguchi, W.Chen, K.Sasakura,c J. Liu, M.Xian, K. Hanaoka, T.Nagano, and F.Ichinose, "Cytoprotective effects of hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonists are mediated by intracellular sulfane sulfur", Medchemcomm., 2014, 5, (10), 1577-1583.
5) N.Fukushima, N.Ieda, M.Kawaguchi, K. Sasakura, T.Nagano, K.Hanaoka, N.Miyata, H.Nakagawa, "Development of photo-controllable hydrogen sulfide donor applicable in live cells", Bioorganic & Medicinal Chemistry Letters., 2015, 25, (2), 175-178.
6) E.Marutani, M.Yamada, T.Ida, K.Tokuda, K.Ikeda, S.Kai, K.Shirozu, K.Hayashida, S.Kosugi, K.Hanaoka, M.Kaneki, T.Akaike, and F.Ichinose,  "Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia", J Am Heart Assoc., 2015, 4, (11), e002125.
"Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia", J Am Heart Assoc., 2015, 4, (11), e002125.
7) K.Hanaoka, K.Sasakura, Y.Suwanai, S.Toma-Fukai, K.Shimamoto, Y..akano, N.Shibuya, T.Terai, T.Komatsu, T. Ueno, Y.Ogasawara, Y.Tsuchiya Y.Watanabe, H.Kimura, C.Wang, M.Uchiyama, H.Kojima, T.Okabe, Y.Urano, T.Shimizu & Tetsuo Nagano, "Discovery and Mechanistic Characterization of Selective Inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide", Scientific Reports 7., 2017, 40227, (10), 1038.
8)Y.Kawahara, Y.Hirashita, C.Tamura, Y.Kudo, K.Sakai, K.Togo, K.Fukuda, O.Matsunari, K.Okamoto, R.Ogawa, K.Mizukami, T.Okimoto, M.Kodama, K.Murakami, "Helicobacter pylori infection modulates endogenous hydrogen sulfide production in gastric cancer AGS cells", Helicobacter, 2020, https://doi.org/10.1111/hel.12732.
Handling and storage condition
| Appearance: | Brown to Greenish brown solid |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| Sensitivity: | To pass test |
| Ambient temperature |












