|
Recent research on cellular senescence shows that senescent cells propagate the effects of aging and contribute to chronic inflammation and diseases such as neurodegeneration. Here are studies that explore the systemic effects of senescence and therapeutic strategies that target senescent cells and their secreted factors. Senescent cells are dysfunctional cells that accumulate in organs and tissues with age and secrete bioactive factors, collectively known as the senescence-associated secretory phenotype (SASP). These factors influence the local microenvironment and can propagate senescence to nearby and distant cells, leading to systemic effects. Systemic senescence contributes to age-related tissue dysfunction, chronic inflammation and the progression of diseases such as cancer, cardiovascular disease and neurodegeneration. Targeting senescent cells or their secreted factors offers a promising therapeutic strategy to attenuate systemic aging and related pathologies. |
|||
|
Hepatocellular senescence induces multi-organ senescence and dysfunction via TGFβ |
Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence |
Senescent cell transplantation into the skin induces age-related peripheral dysfunction and cognitive decline Click here for the original article: Ana Catarina Franco, et. al., Aging Cell, 2024. |
|
|
Point of Interest - Hepatocyte senescence predicts the outcome of acute liver failure, including organ failure, driven by the systemic effects of the TGFβ signaling pathway. - Blocking TGFβ inhibits the spread of senescence and prevents liver-induced kidney dysfunction, highlighting the role of senescence in multi-organ dysfunction. |
Point of Interest - The PPARα agonist fenofibrate restores tissue homeostasis in aged mice and reduces the risk of chronic diseases, suggesting a potential to extend human lifespan. - BMMs are key to senescence propagation, linking aging dysfunction to a treatable mechanism with therapeutic implications.
|
Point of Interest - The senescent fibroblasts in the skin increase mobility and musculoskeletal impairment, demonstrating systemic effects of skin-resident senescence. - Skin senescence correlates with hippocampal dysfunction, linking local senescence to brain dysfunction and age-related cognitive decline. |
|
| Related Techniques | |||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples NEW SPiDER-βGal Blue for fixed cell and for multiple staining with immunostaining and other methods |
||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | ||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Oxygen Consumption Rate(OCR) Plate Assa | Extracellular OCR Plate Assay Kit | ||
| First-time autophagy research | Autophagic Flux Assay Kit | ||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | ||
| Lipid Droplet detection | Lipid Droplet Assay Kit - Blue / Deep Red | ||
| Exosome staining | ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red | ||
| Apoptosis detection in multiple samples | Annexin V Apoptosis Plate Assay Kit | ||
| Cell proliferation/ cytotoxicity assay | Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST | ||
| Related Applications | |||
Co-staining with Lipid droplet and SA-β-Gal in fixed cells |
|||
|
|
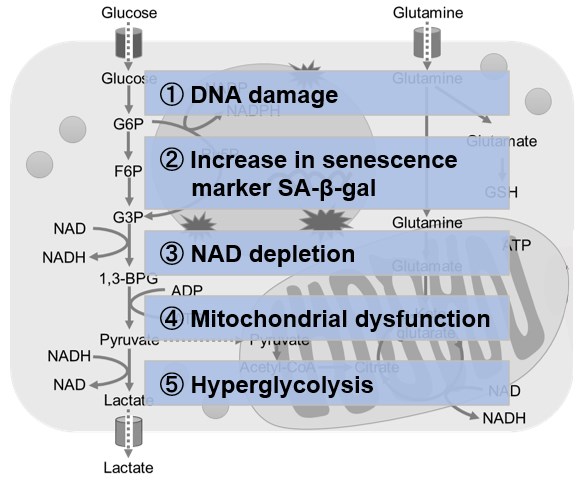
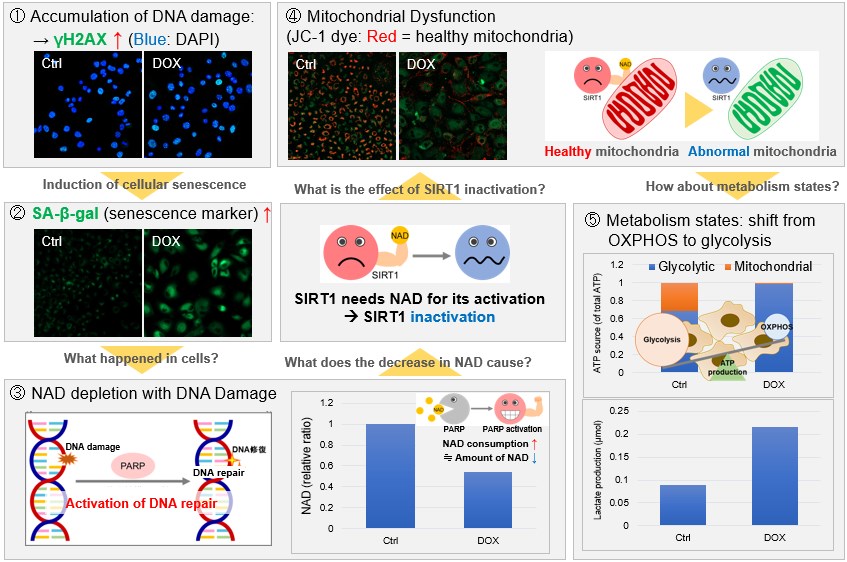
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
||
 |
|||
Multiple staining with oxidative stress-related markers using Doxorubicin-induced senescent cells(flow cytometry) |
|||
|
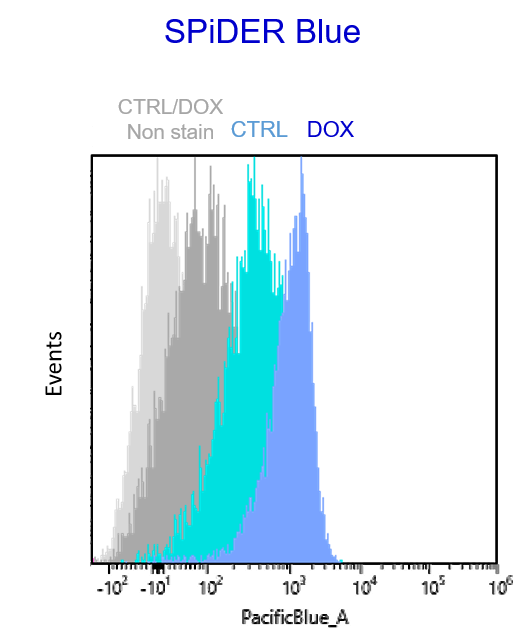
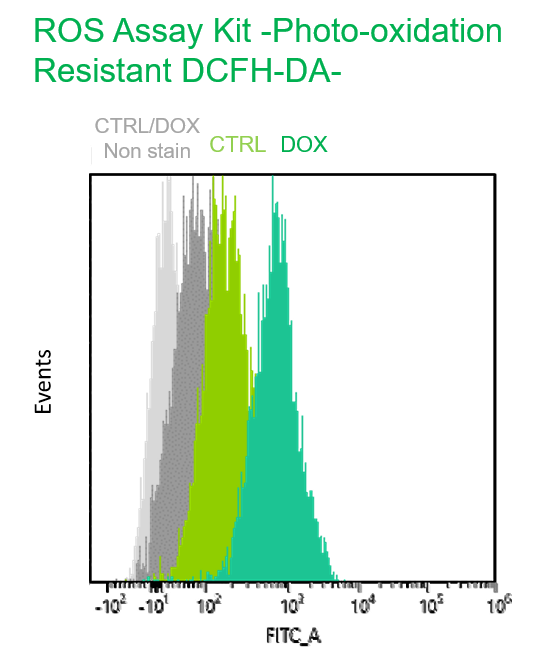
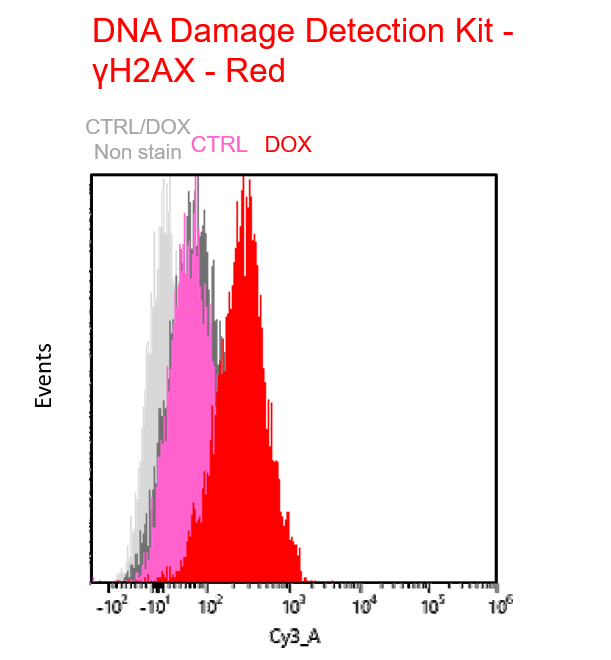
Using A549 cells induced to senescence by doxorubicin (DOX) and normal cells (CTRL), changes in oxidative stress-related markers in senescent cells were analyzed by flow cytometry with multiple staining. SA-βGal as a senescence marker was detected by Cellular Senescence Detection Kit - SPiDER Blue, total ROS as an oxidative stress marker was detected by ROS Assay Kit - Photo-oxidation Resistant DCFH-DA-, and γH2AX as a DNA damage marker was detected by DNA Damage Detection Kit - γH2AX-Red. As a result, total ROS and γH2AX were increased in SA-βGal-positive senescent cells, and the increase in oxidative stress-related markers associated with cellular senescence could be detected by multiple staining. <Experimental Procedure> |
|||