Kalibor

Anion Eliminator
-
Product codeK003 Kalibor
-
CAS No.143-66-8
-
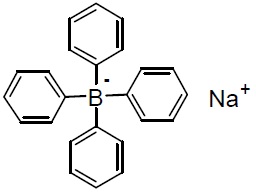
Chemical nameTetraphenylborate, sodium salt
-
MWC24H20BNa=342.22
| Unit size | Price | Item Code |
|---|---|---|
| 25 g | K003-12 | |
| 100 g | K003-14 |
Description
Kalibor is utilized for gravimetric analyses of K+, Rb+, Cs+, NH4+, amine compounds, alkaloids, onium compounds, and other monovalent ions, such as Ag(I), Cu(I) and Tl(I).
Chemical Structure
References
1. R. E. Jensen, et al., Non-aqueous Acids-base Properties of Sodium Tetraphenylboron. Anal Chem. 1972;44:846-848.
2. N. M. Hanken, The Use of Sodium Tetraphenylborate and Sodium Chloride in the Extraction of Fossils from Shales. J Paleontology. 1979;53:738-741.
3. M. Tsubouchi, et al., Determination of Cationic Surfactants by Two-phase Titration. Anal Chem. 1981;53:1957-1959.
4. S. S. Badawy, et al., Potentiometric and Thermal Studies of a Coated-wire Antazoline-selective Electrode. Anal Chem. 1988;60:758-761.
5. K. Vytras, et al., Titrations of Non-ionic Surfactans with Sodium Tetraphenylborate Using Simple Potentiometric Sensors. Analyst. 1989;114:1435-1441.
6. A. Yoo, et al., The Synthesis of Some Lipophilictetra-arylborates for Use in Membrane Electrode Preparation. Talanta. 1991;38:493-496.
Handling and storage condition
| Appearance: | White crystal |
|---|---|
| Purity (Grav.): | ≧ 99.5 % |
| Solubility in water: | To pass test (clear, colorless) |
| Loss on drying (110 °C): | ≦ 0.20 % |
| IR spectrum: | Authentic |
| Solubility in Acetone: | To pass test (clear or almost clear, colorless) |
| Ambient temperature |












