LLPS Forming Condition Screening Kit

Optimization of Conditions for Target Protein Droplets
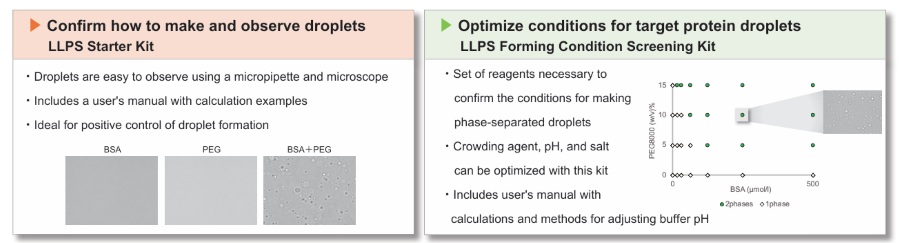
- Set of reagents necessary to confirm the conditions for making phase-separated droplets.
- Crowding agent, pH, and salt can be optimized with this kit
- Includes user's manual with sample calculations and methods for adjusting buffer pH
-
Product codeLL02 LLPS Forming Condition Screening Kit
| Unit size | Price | Item Code |
|---|---|---|
| 300 tests | Find your distributors | LL02-10 |
| 300 tests |
|---|
What is Liquid-Liquid Phase Separation (LLPS)?
Liquid-liquid phase separation (LLPS) is a phenomenon in which certain molecules assemble locally within a cell to form aggregates (droplets) of biomolecules with liquid-like properties. In recent years, LLPS has attracted much attention as it has been shown to affect many biological processes in the cell. Although the study of droplets formed by phase separation is still in its infancy, elucidating how these biological phenomena affect cellular functions and the pathogenesis of disease is considered key to the development of new therapeutic strategies.
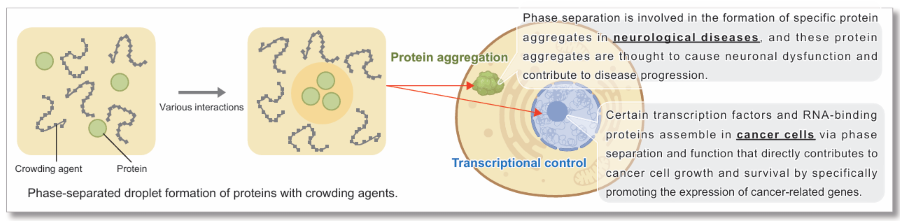
Reference: E. Dolgin, Nature, 2018, DOI: 10.1038/d41586-018-03070-2.
Description
To understand phase separation in living organisms, researchers observe phase-separated droplets in cell-free in vitro systems using purified proteins. However, even proteins that form phase-separated droplets may not do so at certain pH values or salt concentrations. Therefore it is essential to examine of the conditions required for droplet formation. This examination not only verifies whether a protein forms droplets under biochemical conditions but also provides insights into the mechanisms by which droplets are formed. Incidentally, the reduced molecular crowding in extracellular systems compared with intracellular environments can make formation more challenging for droplets. In such cases, adding high-molecular-weight compounds such as PEG as crowding agents can mimic the intracellular crowded environment, thereby facilitating droplet formation.
This kit is a set of crowding agents, buffers, and salts for investigating the conditions required to form phase-separated droplets. By following the protocol provided in this kit, you can determine the phase separation conditions suitable for your proteins and nucleic acids.
*Standard proteins and other controls are not included in this kit.
Manual
Technical info
Generally, when starting LLPS research, it is often necessary to confirm that the target protein forms droplets in a cell-free system.
However, the optimal conditions for this step also differ depending on the protein, and there are various considerations and precautions such as pH, salt type, and the presence or absence of crowding agents*.
We have prepared two types of kits that are ideal for those who are just starting out in LLPS research.
*Crowding agent (molecular adulterant reproducer): A polymer such as PEG or Ficoll that is added to reproduce a crowded intracellular environment.
LL01 LLPS Starter Kit: Ready-to-use kit for the first time LLPS research
LL02 LLPS Forming Condition Screening Kit: Ideal for optimization of conditions for target protein droplets
Optimize droplet forming concentration of BSA with PEG8000 crowding agent
The concentrations of BSA and crowding reagent in droplet formation were investigated. PEG8000 was selected as the crowding agent, and other conditions were 150 mmol/l NaCl, 50 mmol/l HEPES buffer (pH 7.4) (both at final concentration). Plate reader measurements showed a concentration-dependent increase in absorbance (turbidity) at 600 nm for PEG8000 (Figure 1). Visual microscopic droplet mapping showed that droplet formation was observed at all BSA concentrations when 15% PEG8000 was added (Figure 2).
BSA concentrations: 0, 15.6, 31.3, 62.5, 125, 250, 500 µmol/l
PEG8000 conccentrations: 0, 5, 10, 15%.
*For detailed experimental conditions, please refer to Experimental Example 1 in the instruction manual.
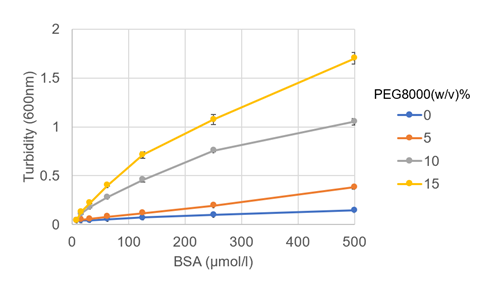
Figure 1: Change in turbidity with BSA and PEG8000 concentrations
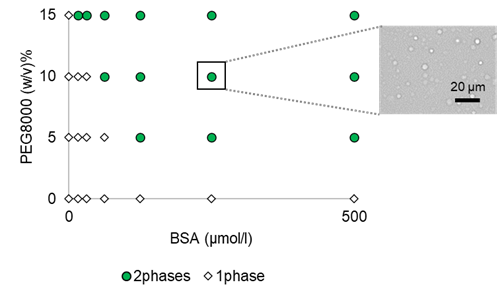
Figure 2: Phase diagram of BSA/PEG droplets and microscopic images of phase-separated droplets
Investigation of the effect of the type of buffer and the pH value
To determine the optimal buffer type and pH for BSA droplet formation, we used 100 µmol/l BSA, 15% PEG8000 as crowding agent, and 150 mmol/l NaCl as salt (all at final concentration). MES, phosphate buffer, HEPES, and Tris-HCl were used as buffers, and conditions were investigated in the pH range of 5.5 - 9.0. The results of turbidity measurement (600 nm absorbance) using a plate reader and visual microscopic observation showed that droplets formed under all conditions, but the lower the pH, the more droplets formed.
*For detailed experimental conditions, please refer to Experimental Example 2 in the instruction manual.
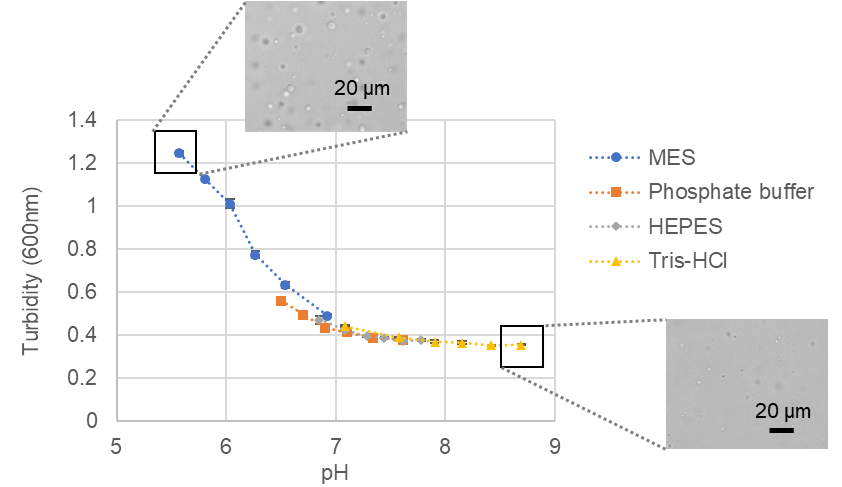
Turbidity changes of phase-separated BSA droplets versus pH and microscopic images
Investigation of salt in droplet formation
The effect of salt type on BSA droplet formation was investigated: BSA was set at 100 µmol/l, the crowding agent was 15% PEG8000, and the buffer was 20 mmol/l HEPES buffer (pH 7.4) (all at final concentration). Three types of salt were used: sodium chloride, potassium chloride, and ammonium sulfate. Turbidity measurements (600 nm absorbance) in a plate reader and visual microscopy showed that the higher the salt concentration, the better the BSA droplets formed. The sodium chloride system showed a trend toward higher turbidity and a greater number of droplets. On the other hand, the system with 500 mmol/l ammonium sulfate showed a lower turbidity value but visually well formed droplets. It is believed that the larger droplets formed compared to the other conditions resulted in lower turbidity values.
*For detailed experimental conditions, see Experimental Experiment 3 in the instruction manual.
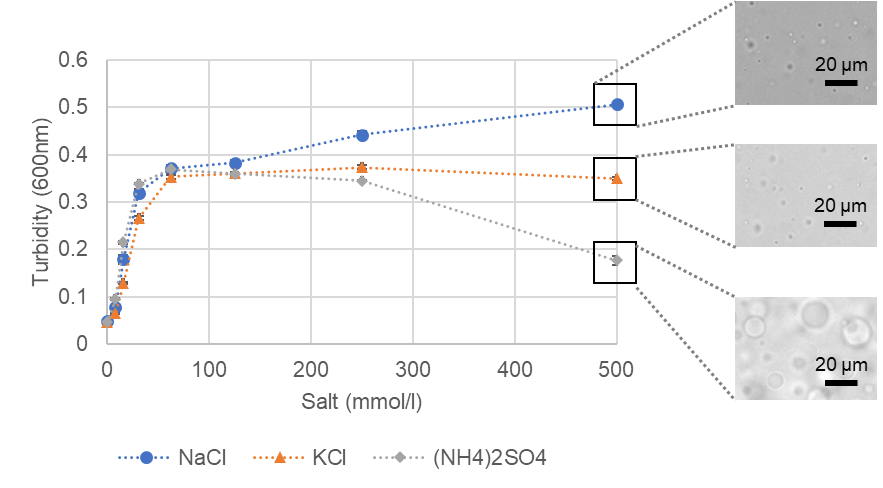
Turbidity changes of phase-separated BSA droplets versus salt concentration and microscopic images
Q & A
-
Q
How many experiments can I run out of a single kit?
-
A
Depending on the concentration setting, 200 µl of a solution with a PEG concentration of 20%, a buffer concentration of 50 mmol/l and a salt concentration of 500 mmol/l can be used for 300 assays. When using a 96-well plate, all the examples of experiments described in the instructions (i.e. crowding agent and protein concentrations, pH and salt concentrations) can be performed with one kit.
-
Q
When performing phase separation experiments, what precautions should I take?
-
A
Please note the following points when performing experiments.
1. The number of pipetting times can affect the droplet formation. The number of pipetting times should be the same for each operation.
2. The number of phase separated droplets may increase or decrease depending on the temperature. It is recommended to keep the room temperature constant for each experiment.
3. The time required for the formation of phase separated droplets after solution preparation depends on the experimental conditions. 10 minutes to 24 hours should be considered as a rough guide.
-
Q
What kind of 96 well plate can I use?
-
A
Use clear bottom or flat bottom plates. For microscopic observation, it is recommended to use glass bottom plates.
-
Q
How should PEG400, PEG4000, and PEG8000 be stored?
-
A
PEG400 is particularly susceptible to moisture absorption. After use, be sure to close the cap and store in the aluminum laminate zip lock bag provided with the silica gel. If the package is to be opened repeatedly, it should be divided into smaller packages and stored in advance.
PEG4000 and PEG8000 also absorb moisture easily, so it is recommended to store them in the aluminum laminated zip lock bags.
-
Q
The PEG4000 has become solid in the container.
-
A
In rare cases, PEG4000 may melt and become solid due to temperature rise during transportation. Even if it becomes solid, its performance as a crowding agent is not affected, so please scrape it off with a spatula or the like. The quality of other components is also unaffected by temperature rise.
-
Q
What wavelength should I use for turbidity measurements in a plate reader?
-
A
To avoid the effects of absorption by the sample, this is often done at wavelengths in the range of 400 to 700 nm. If the sample has a specific absorption wavelength, select a wavelength that avoids that region.
-
Q
Can I expect phase-separated droplets to form when the solution is highly turbid?
-
A
Turbidity also increases as the sample agglomerates. Larger droplet sizes can also result in lower turbidity. To confirm whether phase separation is occurring, microscopic observation is recommended in addition to turbidity measurement.










