NOR 3

NO Detection
-
Product codeN390 NOR 3
-
CAS No.138472-01-2
-
Chemical name(±)-(E)-4-Ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide
-
MWC8H13N3O4=215.21
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | Find your distributors | N390-10 |
Reaction of NO release
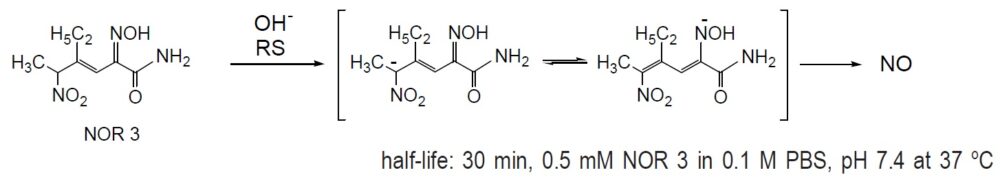
Product Description of NOR Compounds
NORs are ideal NO donors with completely different chemical structures from the other NO donors. Although NORs do not have any ONO2 or ONO moiety, they spontaneously release NO at a steady rate. Even though the NO release mechanism of NOR has not been completely determined, it is confirmed that the byproducts do not possess any significant bioactivities. NOR 3, isolated from Streptomyces genseosporeus, is reported to have strong vasodilatory effects on rat and rabbit aortas and dog coronary arteries. Its activity (ED50=1 nM) is 300 times that of isosorbide dinitrate (ISDN). NOR 3 also increases the plasma cyclic GMP levels, whereas ISDN does not. NOR is a potent inhibitor of platelet aggregation and thrombus formation. NOR 3 (IC50=0-7 mM) effectively inhibits 100% of ADP-initiated human platelet Aggregation, whereas ISDN inhibits only 32% of the total aggregation, even at 100 mM concentrations. NOR 3 has also been reported to have antianginal and cardioprotective effects in the ischemia/reperfusion system. In the rat methacholin-induced coronary vasospasm model, NOR 3 suppressed the elevation of the ST segment dose-dependently and significantly at 1 mg per kg. On the other hand, ISDN suppressed it significantly at 3.2 mg per kg. The difference in the NO release rate of NOR reagents was reflected even on the in vivo hypotensive effects. NOR may also be used orally in a 0.5% methylcellulose suspension. NOR is relatively stable in DMSO solution. NOR 1, which has the shortest half-life, is a promising reagent for making NO standard solutions for calibration. For the preparation of the standard solution, a precisely diluted NOR 1/DMSO solution is added to the buffer solutions.
Technical info
1. Prepare 10 mM NOR stock solution using DMSO. Since the NOR stock solution is not stable, keep it on an ice bath and use it in one day.
2. Add an appropriate volume of the NOR stock solution to the sample solution in which NO is to be released. In order to avoid possible damage to cells by DMSO, the volume of the NOR stock solution should not exceed 1/50 of the sample volume. The sample solution should have sufficient buffering action. NO will be released immediately after the addition of the NOR stock solution.
References
1) Y. Kita, R. Ozaki, S. Sakai, T. Sugimoto, Y. Hirasawa, M. Ohtsuka, H. Senoh, K. Yoshida and K. Maeda, "Antianginal Effects of FK409, a New Spontaneous NO Releaser", Br. J. Pharmacol., 1994, 113, 1137.
2) Y. Kita, Y. Hirasawa, K. Yoshida and K. Maeda, "Antiplatelet Activities of FK409, a New Spontaneous NO Releaser", Br. J. Pharmacol., 1994, 113, 385.
3) M. Hino, M. Iwami, M. Okamoto, K. Yoshida, H. Haruta, M. Okuhara, J. Hosoda, M. Kohsaka, H. Aoki and H. Imanaka, "FK409, a Novel Vasodilator Isolated from the Acid-treated Fermentation Broth of Streptomyces Griseosporeus I. Taxonomy, Fermentation, Isolation, and Physico-chemical and Biological Characteristics", J. Antibiot., 1989 XLII(11), 1578.
4) S. Shibata, N. Satake, N. Sato, M. Matsuo, Y. Koibuchi and R. K. Hester, "Characteristics of the Vasorelaxing Action of (3E)-4-Ethyl-2-hydroxyimino-5-nitro-3-hexamide FK409, a New Vasodilator Isolated from Microbial Sources, in Isolated Rabbit Arteries", J. Cardiovasc. Pharmacol., 1991, 17(3), 508.
5) Y. Kita, Y. Hirasawa, K. Maeda, M. Nishio and K. Yoshida, "Spontaneous Nitric Oxide Release Accounts for the Potent Pharmacological Actions of FK409", Eur. J. Pharmacol., 1994, 257, 123.
6) J.-L. Decout, B. Roy, M. Fontecave, J.-C. Muller, P. H. Williams and D. Loyaux, "Decomposition of FK409, a New Vasodilator: Identification of Nitric Oxide as Metaborite", Bioorg. Med. Chem. Lett., 1995, 5(9), 973.
7) S. Fukuyama, Y. Kita, Y. Hirasawa, T. Azuma, A. Sato, N. Morokoshi, S. Koda, T. Yasuda, S. Oka and H. Sakurai, "A New Nitric Oxide (NO) Releaser: Spontaneous NO Release from FK409", Free Rad. Res., 1995, 23(5), 443.
8) Y. Kita, K. Ohkubo, Y. Hirasawa, Y. Katayama, M. Ohno, S. Nishino, M. Kato and K. Yoshida, "FR144420, a Novel, Slow, Nitric Oxide-releasing Agent", Eur. J. Pharmacol., 1995, 275, 125.
9) M. Kato, S. Nishino, M. Ohno, S. Fukuyama, Y. Kita, Y. Hirasawa, I. Nakanishi, H. Takasugi and K. Sakane, "New Reagents for Controlled Release of Nitric Oxide. Structure-stability Relationships", Bioorg. Med. Chem. Lett., 1996, 6(1), 33.
10) Y. Kita, Y. Hirasawa, S. Fukuyama and K. Yoshida, "FK409, a Novel Spontaneous NO Releaser: Comparative Pharmacological Studies with ISDN", Cardiovasc. Rrug. Revi., 1996, 14(2), 148.
11) Y. Hirasawa, T. Sugimoto, S. Fukuyama, Y. Kato, H. Takamatu, M. Ohno, S. Nishino, M. Kato, K. Maeda, J. Seki and Y. Kita, "Antianginal Effects of FR144420, a Novel Nitric Oxide-releasing Agent", Eur. J. Pharmacol., 1996, 303, 55.
12) M. Sato and M. Kawatani, "Nitric Oxide Raises Cytosolic Concentrations of Ca2+ in Cultured Nodose Ganglion Neurons from Rabbits", Neurosci. Lett., 1996, 206, 69.
13) Y. Kita, Y. Hirasawa, S. Fukuyama, K. Ohkubo, Y. Kato, H. Takamatsu, M. Ohno, S. Nishino, M. Kato and J. Seki, "Oral Biological Activities of Spontaneous Nitric Oxide Releaser are Accounted for by their Nitric Oxide-releaseing Rates and Oral Absorption Manners", J. Pharmacol. Exp. Ther., 1996, 276(2), 421.
14) S. Fukuyama, Y. Hirasawa, Y. Kato, M. Nishino, M. Ohno, S. Nishino, K. Maeda, M. Kato and Y. Kita, "Structure-activity Relationships of Spontaneous Nitric Oxide Releasers, FK409 and its Derivatives", J. Pharmacol. Exp. Ther., 1997, 282(1), 236.
15) Y. Kita, Y. Hirasawa, Y. Kato, K. Ohkubo, M. Ohno, S. Nishino, M. Kato and S. Fukuyama, "Comparison of Hemodynamic Effects of Nitric Oxide (NO) Donors with Different NO-releasing Properties in Rats", J. Cardiovasc. Pharmacol., 1997, 30(2), 223.
16) Y. Hirasawa, Y. Kato, S. Fukuyama, M. Ohno, S. Nishino, M. Kato and Y. Kita, "Comparison of Antiplatelet Effect of two Nitric Oxide-donating Agents, FR146801 and FK409", Thrombosis Haemost., 1998, 79, 620.
Q & A
-
Q
NOCとNORは両方ともNO発生剤として使用されますが、どう違うのですか?
-
A
構造的には全く異なるタイプのNOドナーです。
性能の違いとしては
【NOC】
高水溶性
水溶液での調製が容易(NaOH溶液)
NOCは高pH(アルカリ性)ほどNO放出が遅くなる。半減期は(37℃、pH7.4)
NOC7(5min) > NOC5(25min) > NOC12(100min) > NOC18(21hrs)【NOR】
比較的水溶性が低い
有機溶媒(DMSO)溶液の方が安定に扱える。
経口投与が可能である。
NORは低pH(酸性)ほどNO放出が遅くなる。半減期は(37℃、pH7.4)
NOR1(1.8min) > NOR3(30min) > NOR4(60min) > NOR5(20hrs)
-
Q
NOR類を使用する際の溶解する溶媒は何がいいでしょうか?
-
A
乾燥DMSOをお使いください。
乾燥DMSOに溶解してストック溶液とし、所定量を試料溶液に加えます。
ストック溶液とした場合、一両日中にご使用下さい。
NO発生は事実上中性バッファー(サンプル溶液)に添加された時点から開始されます。
生理食塩水などの緩衝作用のないものでは、NOはきれいに放出しませんのでご注意ください。
-
Q
NOR3の溶解性を教えてください。
-
A
NORs、NOCsの溶解性は以下の通りです。
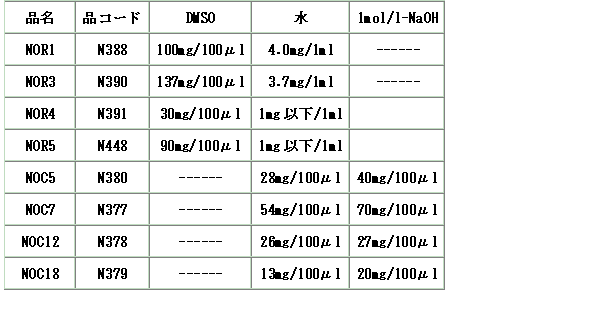
*注意*
NORsは水にも溶けますが、ストック液の調整は「DMSO」で行ってください。
中性の水では溶解直後から分解(NOの放出)が始まります。
-
Q
How do I prepare a stock solution?
-
A
Prepare 10-50 mM NOR solution with DMSO. The DMSO should be dried. Then add enough NOR solution to the cell culture to obtain a suitable concentration of NOR.
-
Q
What is the solubility of NOR compounds?
-
A
NOR 1: 100 mg per 100 ml DMSO (4.3 M)
NOR 3: 137 mg per 100 ml DMSO (6.4 M)
NOR 4: 30 mg per 100 ml DMSO (1.0 M)
NOR 5: 30 mg per 100 ml DMSO (0.9 M)
-
Q
Is oral administration possible?
-
A
Yes. Please review the article by Kita and colleagues (Eur. J. Pharmacol., 257, 123-130, 1994).
-
Q
How many NO molecules does each NOR molecule release in physiological conditions? What are the byproducts?
-
A
On average, each NOR molecule releases from 1 to 1.5 NO molecules in physiological conditions. Unfortunately, the structure of NOR byproducts remains unclear. However, the NOR byproducts have no cytotoxicity at the normal concentration for NO release experiments.
Handling and storage condition
| Appearance: | White crystalline powder |
|---|---|
| Purity (HPLC): | ≧ 98.0 % |
| Solubility in Dimethyl sulfoxide: | To pass test (clear, colorless to pale yellow) |
| -20°C | |
|
Danger / harmful symbol mark |

|
|---|---|










