S-Nitrosoglutathione

NO Detection
-
Product codeN415 S-Nitrosoglutathione
-
CAS No.57564-91-7
-
Chemical nameN-(N-L-γ-Glutamyl-S-nitroso-L-cysteinyl)glycine
-
MWC10H16N4O7S=336.32
| Unit size | Price | Item Code |
|---|---|---|
| 25 mg | Find your distributors | N415-10 |
| 100 mg | Find your distributors | N415-12 |
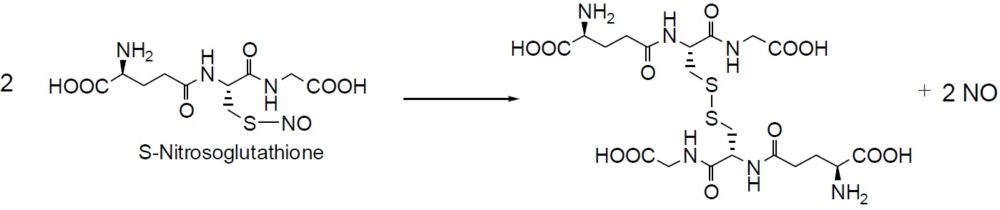
Reaction of NO release
Product Description of S-Nitrosothiols
Nitrosothiol compounds release NO and become disulfides under specific physiological conditions. While most of the S-nitrosothiol compounds are unstable, S-Nitrosoglutathione is exceptionally stable. Furthermore, S-Nitrosoglutathione is water-soluble. Although S-nitrosothiol is a good NO donor with no nitrate tolerance, there is evidence that S-nitrosothiol itself has NO-like activity during guanylate cyclase activation. Another important reaction of nitrosothiol is NO transfer to other thiol compounds. Since it depends on the pKa of thiols, this transfer reaction proceeds at physiological pH levels. The relaxation efficiency of these nitrosothiol has been compared using rataprta ring samples:SNAP > S – N i t r o s o g l u t a t h i o n e = S – N i t r o s o – N -acetylcysteine>S-Nitrosocoenzyme A>S-Nitroso-L-cysteine. Dr. Kowaluk and others reported that the spontaneous liberation of NO from SNAP could not account for in vitro vascular relaxation. The spontaneous release of NO from nitrosothiol compounds may not be a key element of vascular relaxation. Metabolites of nitrosothiol generated at the cell membrane might be the essential element for relaxation.
References
1) A. Gibson, R. Babbedge, S. R. Brave, S. L. Hart, A. J. Hobbs, J. F. Tucker, P. Wallace and P. K. Moore, "An Investigation of Some S-Nitrosothiols, and of Hydroxy-arginine, on the Mouse Anococcygeus", Br. J. Pharmacol., 1992, 107, 715.
2) M. W. Radomski, D. D. Rees, A. Dutra and S. Moncada, "S-Nitroso-glutathione Inhibits Platelet Activation in Vitro and in Vivo", Br. J. Pharmacol., 1992, 107, 745.
3) R. M. Clancy and S. B. Abramson, "Novel Synthesis of S-Nitrosoglutathione and Degradation by Human Neutrophils", Anal. Biochem., 1992, 204, 365.
4) J.-W. Park, G. E. Billman and G. E. Means, "Transnitrosation as a Predominant Mechanism in the Hypotensive Effect of S-Nitrosoglutathione", Biochem. Mol. Biol. Int., 1993, 30(5), 885.
5) D. Barrachina, S. Calatayud, J. Esplugues, Brendan J. R. Whittle, S. Moncada and J. V. Esplugues, "Nitric Oxide Donors Preferencially Inhibit Neuronally Mediated Rat Gastric Acid Secretion", Eur. J. Pharmacol., 1994, 262, 181.
6) E. A. Konorev, M. M. Tarpey, J. Joseph, J. E. Baker and B. Kalyanaraman, "S-Nitrosoglutathione Improves Functional Recovery in the Isolated Rat Heart After Cardioplegic Ischemic Arrest-evidence for a Cardioprotective Effect of Nitric Oxide", J. Pharmacol. Exp. Ther., 1995, 274(1), 200.
7) S. C. Askew, D. J. Banett, J. McAninly and D. L. H. Williams, "Catalysis by Cu2+ of Nitric Oxide Release from S-Nitrosothiols (RSNO)", J. Chem. Soc. Perkin Trans.2, 1995, 741.
8) D. J. Banett, A. Rios and D. L. H. Williams, "NO-group Transfer(Transnitrosation) between S-Nitrosothiols and Thiols. Part 2", J. Chem. Soc. Perkin Trans.2, 1995, 1279.
9) J. G. D. Man, B. Y. De. Winter, G. E. Boeckxstaens, A. G. Herman and P. A. Pelckmans, "Effect of Cu2+ on Relaxations to the Nitrergic Neurotransmitter, NO and S-Nitrosothiols in the Rat Gastric Fundus", Br. J. Pharmacol., 1996, 119(5), 990.
10) A. P Dicks and D. L. H. Williams, "Generation of Nitric Oxide from S-Nitrosothiols Using Protein-bound Cu2+ Sources", Chem. Biol., 1996, 3, 655.
11) J. A. Cook, S. Y. Kim, D. Teague, M. Murali, C. Krishna, R. Pacelli, A. M. Miles, M. B. Grisham and D. A. Wink, "Convenient Colorimetric and Fluorometric Assays for S-Nitrosothiols", Anal. Biochem., 1996, 238, 150.
12) S. X. L. Liu, Bo Xuan, Z. Chen, R. S. Varma and G. C. Y. Chiou, "Nitric Oxide Donors: Effects of S-Nitrosoglutathione and 4-Phenyl-3-furoxancarbonitrile on Ocular Blood Flow and Retinal Function Recovery", J. Ocul. Pharmacol. Therpeut., 1997, 13(2), 105.
13) C. Alpert, N. Ramdev, D. George and J. Loscalzo, "Detection of S-Nitrosothiols and Other Nitric Oxide Derivatives by Photolysis-chemiluminescence Spectrometry", Anal. Biochem., 1997, 245, 1.
14) T. Akaike, K. Inoue, T. Okamoto, H. Nishino, M. Otagiri, S. Fujii and H. Maeda, "Nanomolar Quantification and Identification of Various Nitrosothiols by High Performance Liquid Chromatography Coupled with Flow Reactors of Metals and Griess Reagent", J. Biochem., 1997, 122, 459.
Handling and storage condition
| Appearance: | Pink powder |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| Solubility in water: | To pass test (clear, pink yellow) |
| Solubility in Dimethyl sulfoxide: | To pass test (clear, pink yellow) |
| -20°C |










