n-Octyl-β-D-glucoside

Detergent
-
Product codeO001 n-Octyl-β-D-glucoside
-
CAS No.29836-26-8
-
Chemical nameN-Octyl-β-D-glucopyranoside
-
MWC14H28O6=292.37
| Unit size | Price | Item Code |
|---|---|---|
| 1 g | O001-10 | |
| 5 g | O001-12 |
Introduction
The phospholipid bilayer is the basic structure of the cell membrane. The most important functions of cells include transportation of substances, energy exchange, and transmission of information. These functions are conducted at the cell membrane by membrane proteins. In membrane biochemistry research, membrane proteins are solubilized and purified to study their structure and function. Proteins bound to cell membranes have hydrophobic sites buried within the phospholipid bilayers and hydrophilic sites facing toward the water layer. Detergents are used to isolate large insoluble molecules such as proteins. Detergents interact with the hydrophobic sites of proteins, which are then solubilized in the water layer, thus separating membrane proteins. It is important to choose a detergent that does not disrupt the bioactivities of target proteins. A detergent requires the following characteristics to be suitable for isolation of
membrane proteins:
1. Sufficient protein solubilization capability
2. No denaturing or inactivation of proteins
3. No interference with protein activities
4. No precipitation at 4℃
5. Appropriate critical micelle concentrations (CMC) and micelle size
6. No absorption in the UV region
7. No toxicity
8. Availability of detergent detection methods
9. Non-ionic detergent if ion exchange chromatography is used
In the past, polyoxyethylene ether non-ionic detergents were widely used. These detergents, however, had several problems, such as denaturation of proteins and low CMC value, which cannot be separated easily by dialysis. n-Octyl-β-D-glucoside, n-Octyl-β-Dthioglucoside, CHAPS, and CHAPSO eliminate these problems and are widely used today. Most of the current detergents are non-ionic and easily applied to ion exchange chromatography purification. deoxy-BIGCHAP is a non-ionic detergent possessing deoxycholic acid and a gluconamide polar group. It has a high CMC value of 1.4 mM and can be easily separated by dialysis. Because its UV absorbance is low, it can be used for the determination of proteins. deoxy-BIGCHAP has been used for the extraction of opioid receptors from neuroblastoma or hybrid cells of glyoma. It has also been applied to adenylate cyclase or acetyltransferase. These detergents are also widely used to solubilize chromophores or to stabilize enzymes in diagnostic analyses and biochemical assays.
Trials of various kinds of detergents are needed to find the appropriate detergent for each study. Dojindo’s Detergent Screening Sets, which contain assorted packages of detergents, are available for use in these trials.
Technical info
Detergents are amphipathic compounds, with both lipophobic and lipophilic sites, that will form micelles above a critical concentration that is specific to each detergent. This is called the critical micelle concentration (CMC). The solubilizing abilities of detergents increase dramatically above their CMC values. After extracting membrane proteins, detergents can be easily removed by dilution, and then dialysis.
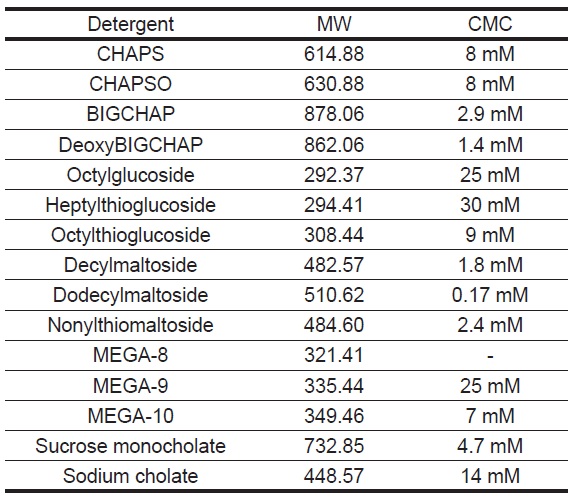
Table 1 Molecular Weight and Critical Micelle Concentration of Detergents
References
1. A. Levitzki, Reconstitution of Membrane Receptor Systems. Biochim Biophys Acta. 1985;822:127-153.
2. S. Horiuchi, et al., Characterization of a Membrane-Associated Receptor from Ratsinusoidal Liver Cells That Binds Formaldehyde-Treated Serum Albumin. J Biol Chem. 1985;260:475-481.
3. H. Tokuda, et al., Reconstitution of Translocation Activity for Secretory Proteins from Solubilized Components of Escherichia coli. Eur J Biochem. 1990;192:583-589.
4. K. Kameyama, et al., Micellar Properties of Octylglucoside in Aqueous Solutions. J Colloid Interface Sci. 1990;137:1-10.
5. M. Nishikawa, et al., Decreased Expression of Type II Protein Kinase C in HL-60 Variant Cells Resistant to Induction of Cell Differentiation by Phorbol Diester. Cancer Res. 1990;50:621-626.
6. H. Tokuda, et al., Purification of SecE and Reconstitution of SecE-dependent Protein Translocation Activity. FEBS Lett. 1991;279:233-236.
Handling and storage condition
| Appearance: | White powder |
|---|---|
| Purity (GC): | ≧ 98.0 % |
| Solubility in water: | To pass test (clear to almost clear, colorless) |
| Specific rotation (20°C): | -32.0~-29.0o・cm2・dag-1 |
| IR spectrum: | Authentic |
| 0-5°C |












