Dojindo Laboratories have developed and improved detection methods to evaluate the nutritional uptake capacity of cells. Here, we present useful advice from advanced researchers who have used our products.
Cellular Uptake Assay Data Collection
・All data are listed in the following table: Click the link in the table to view the data in detail
|
Uptake Nutrition |
Cell Line |
Reagents/Stimulation, etc., |
Data Source |
|---|---|---|---|
| Glucose | Human liver cancer cell line (HepG2 cell) |
Glucose transporter inhibitor (Cytochalasin B) |
Dojindo Laboratories |
| Glucose | Mouse colon cancer cell line (Colon26 cell) |
Boric acid, Glucosamine, 2-Deoxy glucose |
Prof. Hiroshi Maeda(Founder of BioDynamics Laboratory Inc.) |
| Glucose | Mouse lung cancer cell line (3LL cell) |
Glycolysis inhibited reagents | Prof. Heiichiro Udono (Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences) |
| Glucose | Astrocytoma Cell Line (U-251MG cell) |
X-ray Irradiation | Assistant Prof. Shinya Kato(Mie University Advanced Science Research Promotion) |
| Glucose | Mouse Activated T Cell (CD4+T cell) |
Glycolytic enzyme Pgam1-deficient cells | Prof. Masakatsu Yamashita (Ehime University Department of Immunology, Graduate School of Medicine) |
| Glucose | Mouse Progenitor Adipocytes (3T3L1 cell) |
Differentiation of mouse progenitor adipocytes into adipocytes | Associate Prof. Yoshihiro Matsumura (Tokyo University, Research Center for Advanced Science and Technology) |
| Glucose | Mouse Preadipocyte Cell (3T3L1 cell) |
Insulin | Dojindo Laboratories |
| Amino Acids | Human Breast Cancer Cells (MCF7 cell) |
WT & LAT-1 Overexpressed Cell | Lecturer Yasuhiro Saito (Keio University, Institute for Advanced Biosciences) |
| Amino Acids | Human Cervical Cancer Cells (HeLa cell) |
LAT Inhibitor (BCH: 2-Aminobicyclo[2.2.1]heptane-2-carboxylic acid) |
Dojindo Laboratories |
| Amino Acids | Mouse Myoblasts (C2C12 cell) |
Egg white peptide | Prof. Mamoru Matsubara (Gifu University of Medical Science) |
| Cystine | Human Astrocytoma (U251 cell) |
WT & xCT knockdown Cell | Assistant Prof. Hironori Kato (Kyoto University, Graduate School of Biostudies) |
| Cystine | Human Lung Cancer Cell (A549 cell) Human Colon Cancer Cells(HCT116 cell) |
xCT Inhibitor (Sulfasalazine) | Prof. Hideyuki Saya (Keio University, Institute for Advanced Biosciences) |
| Cystine | Human Sarcoma Cells (HT1080 cell) |
WT, xCT knockdown, xCT Overexpressed Cell |
Prof. Hideo Sato (Niigata University, School of Health Sciences) |
| Cystine | Human Liver Cancer Cell (HepG2 cell) |
Diethyl maleate | Dojindo Laboratories |
|
Glucose, Amino Acids, |
Human Lung Cancer Cell (A549 cell) |
xCT Inhibitor (Erastin) | Dojindo Laboratories |
Introduction
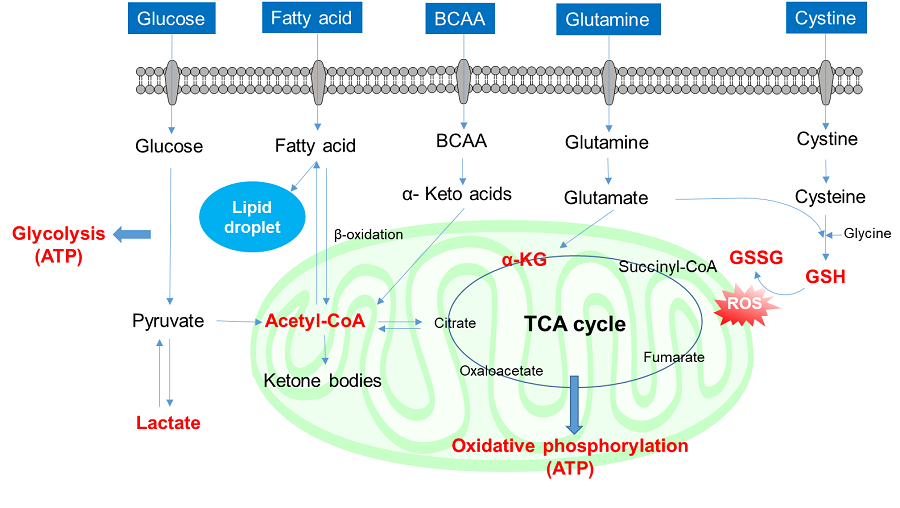
Cells absorb a variety of nutrients (glucose, fatty acids, and amino acids, amongst others) and contribute to the production of proteins, lipids, nucleic acids, and energy necessary for cell proliferation and survival, as well as the maintenance of intracellular redox through intracellular metabolism. These nutrient metabolism processes vary depending on the extracellular environment, the state of the cell, and the cell type. Recently, it has become clear that nutrient metabolism is involved in the regulation of various cellular functions, including gene expression, and has thus attracted much attention.
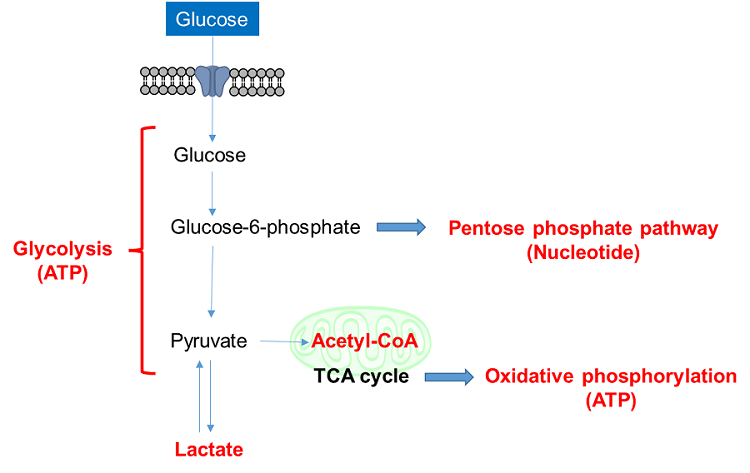
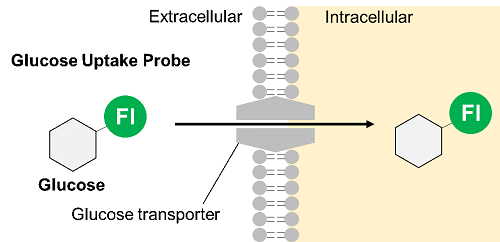
1. Glucose uptake and intracellular metabolism
Glucose is one of the most important nutrients and is taken up into cells mainly via glucose transporters (GLUTs ). Intracellular glucose is a major source of ATP, nucleic acids, and lipids. After glucose is taken into the cell, it is metabolized by various enzymes and is converted to pyruvate via the glycolytic pathway. Then pyruvate participates in the citric acid circuit (TCA cycle ) via acetyl-CoA in the mitochondria and is used to produce NADH and FADH, which are necessary for ATP production. Although ATP can be synthesized by both the glycolysis pathway and the OXPHOS pathway, OXPHOS using NADH and FADH in mitochondria is the most efficient and major ATP production pathway.
Cancer cells uptake large amounts of glucose to maintain their high proliferative capacity. Because cancer cells mainly use the glycolytic pathway to produce ATP, it has been a classic anti-cancer drug target. Although effective anticancer drugs have not yet been developed, the glycolytic pathway is still a major drug target in cancer cells and is the most important pathway for understanding cancer cell metabolism.
1-1. Glucose uptake assay
Radiolabeled glucose is the most common method of evaluating the glucose uptake capacity of cells. However, because of the radioactive compound, it is complicated to perform and lacks versatility. An enzymatic cycling method using 2-deoxy-D-glucose (2-DG ) can be used for colorimetric or fluorescent plate assays, but it is not applicable for cell imaging or flow cytometry. The fluorescent glucose tracer 2-NBDG has recently been more widely used, but its low fluorescence intensity is widely criticized.
Thus, Dojindo Laboratories have developed a new fluorescent-labeled glucose derivative, Glucose Uptake Probe (Blue, Green, Red), to replace 2-NBDG. The Glucose Uptake Probe is much brighter than 2-NBDG and can measure the glucose uptake capacity of cells with high sensitivity. In addition, there are three different colors to meet different needs, enabling its use in various applications.

|
Category |
Product Name(Click the links in the table to view the product details) |
|---|---|
|
Glucose Uptake |
1-2. Application: Glucose uptake of cancer cells
Inhibition of glucose uptake by glucose transporter inhibitors
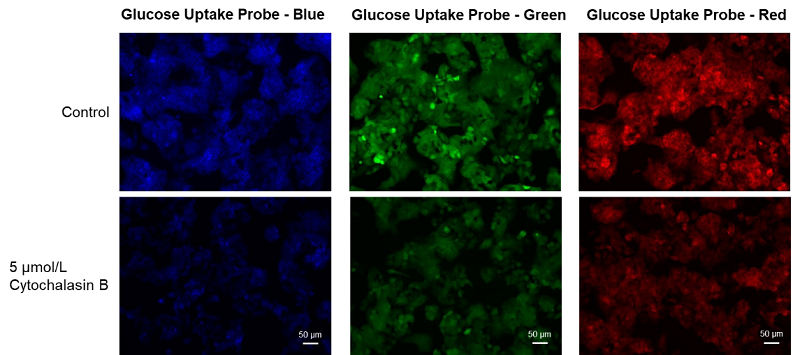
Cell line: Human liver cancer cell, HepG2
Stimulation: Glucose transporter inhibitor, cytochalasin B
HepG cells (human hepatocellular carcinoma cells) were treated with cytochalasin B and the changes in the glucose uptake capacity of the cells were observed by fluorescence microscopy using the Glucose Uptake Probe. This approach confirmed that the glucose uptake ability of the cells was inhibited by cytochalasin B treatment.
Glucose uptake inhibition by reagents
Provided by Prof. Hiroshi Maeda (Founder of BioDynamics Laboratory Inc.)
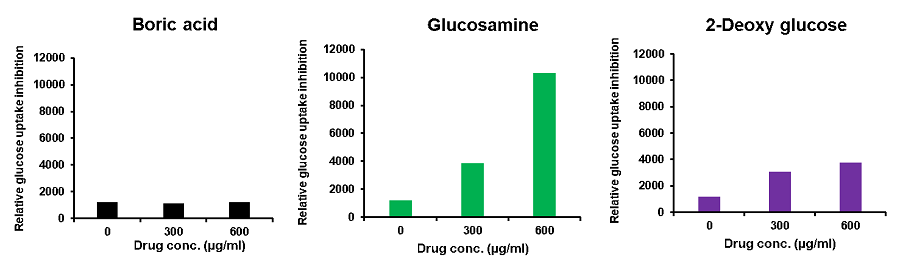
Cell line: Mouse colon cancer cells, Colon26
Reagents: Boric acid, glucosamine, 2-deoxy-D-glucose
Colon26 cells (mouse colon cancer cells) were treated with various reagents, and the glucose uptake of the cells was measured using the Glucose Uptake Probe-Green. The results demonstrated that glucose uptake was inhibited by the addition of the reagents, and the effect varied depending on these reagents.
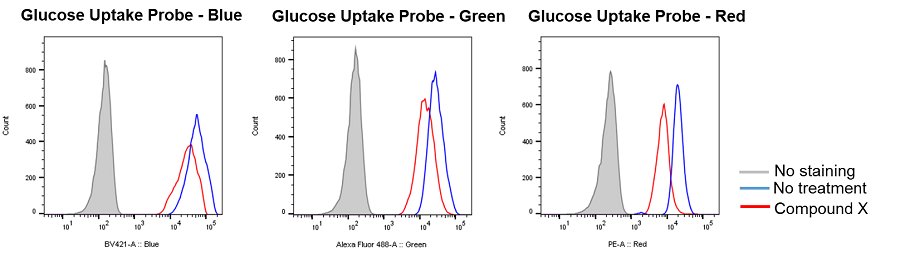
Inhibition of glucose uptake by compounds that inhibit the glycolytic pathway
Provided by Prof. Heiichiro Udono (Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences)
Cell line: Mouse lung cancer cells, 3LL
Reagents: Glycolytic pathway inhibitor (not disclosed, referred to as Compound X)
3LL cells were treated with Compound X, which inhibits the glycolytic pathway, and the glucose uptake of the cells was measured by flow cytometry using the Glucose Uptake Probe. The results demonstrated that the glucose uptake of the cells was inhibited by Compound X.
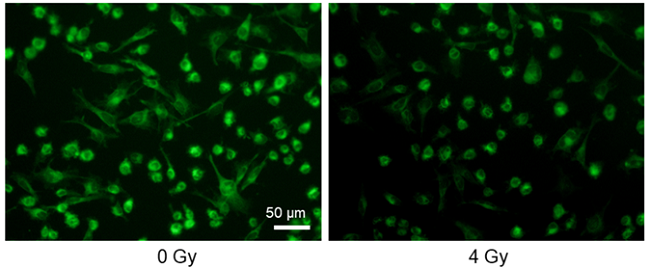
Decreased glucose uptake by X-ray irradiation
Provided by Assistant Prof. Shinya Kato (Mie University Advanced Science Research Promotion)
Cell line: Astrocytoma cell line, U-251MG
Stimulation: X-ray irradiation
X-ray irradiation of U-251MG cells decreased the glucose uptake capacity of the cells.
1-3. Application: Glucose uptake by immune cells
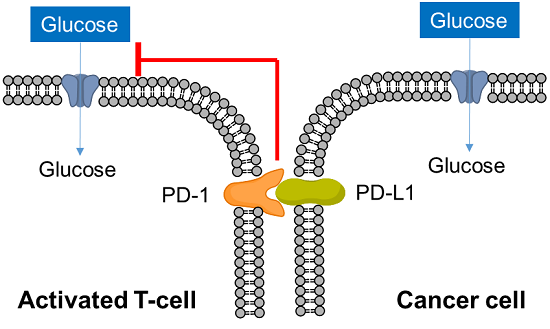
T cells play a central role in the immune system to eliminate cancer cells. In recent years, it has become clear that metabolism is also involved in the regulation of T cell functions such as differentiation and activation, and research on metabolism in cancer immunity has become more popular. Cancer cells absorb large amounts of nutrients to maintain their proliferative activity, while activated T cells also require large amounts of nutrition, especially glucose, to eliminate cancer cells. Therefore, activated T cells and cancer cells compete for glucose uptake.
Cancer cells express PD-L1 to suppress T cell activity in response to PD-1 on the surface of activated T cells, an immune checkpoint molecule, and recent studies have shown that this interaction suppresses T cell glucose uptake. Cancer cells also regulate the metabolism of immune cells to evade the immune system in this way, and it is important to understand the metabolism of immune cells as well as cancer cells in the field of cancer immunology.
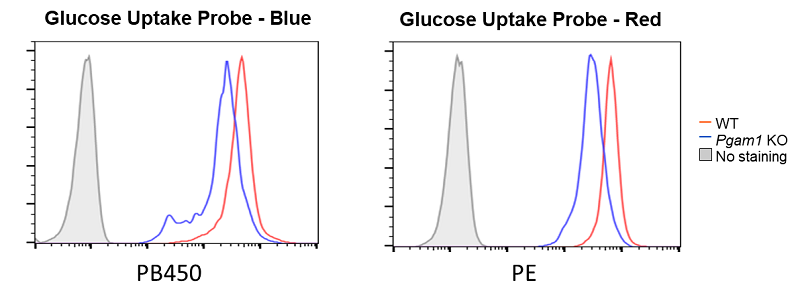
Decreased glucose uptake due to deficiency of the glycolytic enzyme Pgam1
Provided by Prof. Masakatsu Yamashita (Ehime University Department of Immunology, Graduate School of Medicine)
Cell line: Mouse activated T cells, CD4+ T cell, with deficiency of the glycolytic enzyme Pgam1
Decreased glucose uptake was observed in Pgam1-deficient CD4+ T cells (mouse activated T cells).
1-4. Application: Glucose uptake in adipocytes
Diabetes is a disease in which glucose in the blood is not taken up into the cells, resulting in increased blood glucose levels. In muscle cells and adipocytes, insulin stimulation causes GLUT4, a glucose transporter, to migrate to the surface of the cell membrane and promote glucose uptake. This insulin-responsive glucose uptake is closely related to diabetes and is an important target in the clarification of diabetes and the development of therapies.
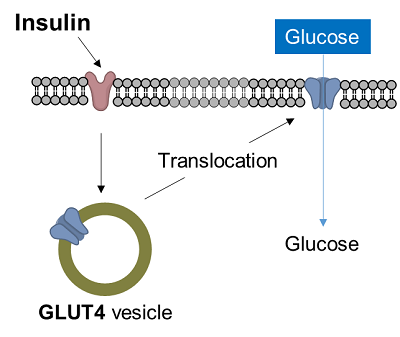
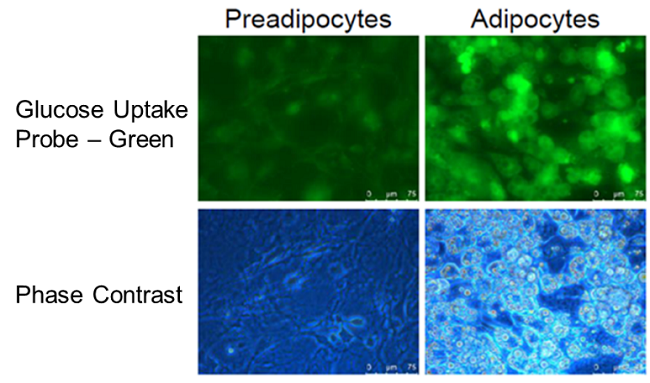
Enhancement of glucose uptake during differentiation into adipocytes
Provided by Associate Prof. Yoshihiro Matsumura (Tokyo University, Research Center for Advanced Science and Technology)
Cell line: Mouse progenitor adipocytes, 3T3L1
The glucose uptake capacity of mouse progenitor adipocytes increased as long as differentiation into adipocytes was observed.
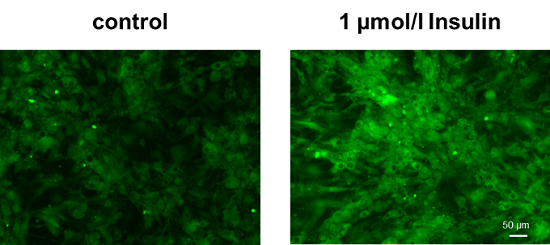
Enhancement of glucose uptake in adipocytes after insulin treatment
Cell line: Mouse progenitor adipocytes, 3T3L1
Insulin treatment of adipocytes differentiated from mouse progenitor adipocytes enhanced glucose uptake.
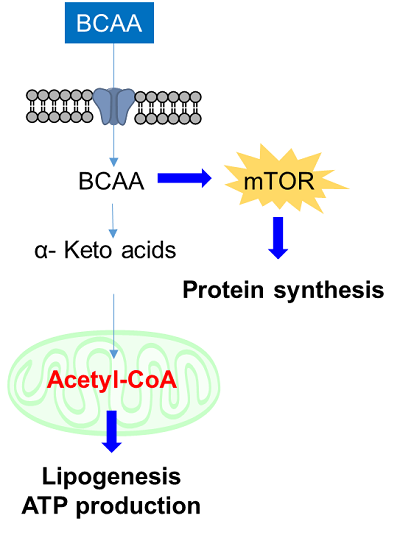
2. Amino acids uptake and intracellular metabolism
Amino acids are essential for intracellular energy production and the synthesis of proteins, lipids, and nucleic acids, and are taken up into cells mainly via transporters. Cancer cells take up large amounts of amino acids as well as saccharides and fatty acids to maintain cell proliferation and enhanced intracellular metabolism, and amino acid transporters are attracting attention as targets for cancer diagnosis and drug discovery. For example, L-type amino acid transporter 1 (LAT1) is expressed in many cancer cells and is known to take up essential amino acids including branched-chain amino acids (BCAA). In recent years, BCAAs in particular have attracted attention as essential nutrients involved in major protein synthesis and energy production in cancer cells.
2-2. Amino acids uptake assay
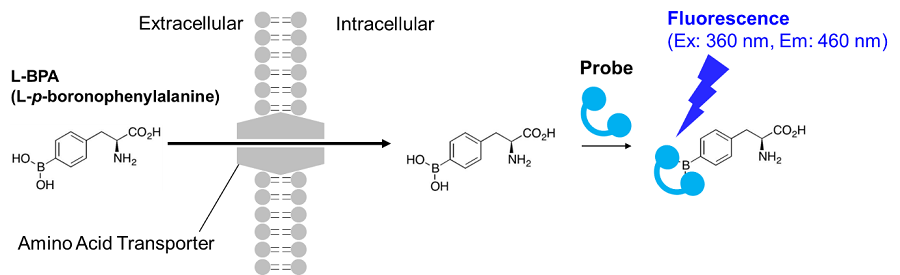
Radioisotope-labeled amino acid assays have been used to evaluate amino acid transporters for many years. However, the use of radioisotopes makes it cumbersome to handle, and it is not widely used. Therefore, Dojindo Laboratories have developed a new method using a fluorescent probe developed by Kirihata et al. L-boronophenylalanine (L-BPA) used in this method is an amino acid analog used in boron neutron capture therapy (BNCT), a cancer treatment. It is known to be taken up into cells via the amino acid transporter large neutral amino acid transporter (LAT). BPA taken up into cells can be detected (λex=360 nm, λem=460 nm) by probes that specifically bind to BPA and emit fluorescence. This technique can be applied to fluorescence imaging, plate reader measurement, and flow cytometry, and is useful for evaluating the amino acid uptake capacity of cells and screening amino acid transporter inhibitors.
|
Category |
Product Name (Click the link in the table to view the details) |
|---|---|
|
Amino Acid Uptake |
2-3. Application: Amino acids uptake
Enhanced amino acid uptake in LAT1-overexpressing cells
Provided by Lecturer Yasuhiro Saito (Keio University, Institute for Advanced Biosciences)
Cell line: MCF7 cells
The amino acid uptake of LAT-1-overexpressing MCF7 cells was greatly increased compared with wild type MCF7 cells.

Inhibition of amino acid uptake by LAT1 inhibitor BCH
Cell line: Human cervical cancer cells, HeLa
Inhibitor: LAT1 Inhibitor, BCH (2-aminobicyclo[2.2.1]heptane-2-carboxylic acid), and L-leucine
The intracellular uptake of BPA was inhibited by the addition of BCH, a LAT inhibitor, and L-leucine, a BCAA.
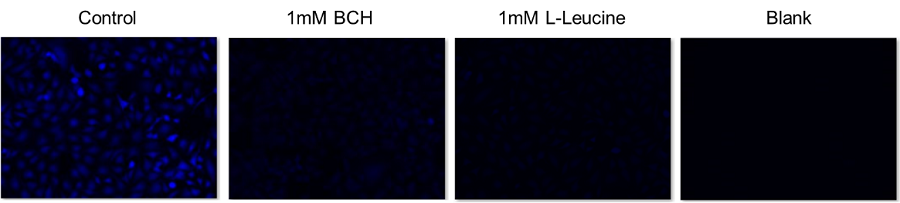
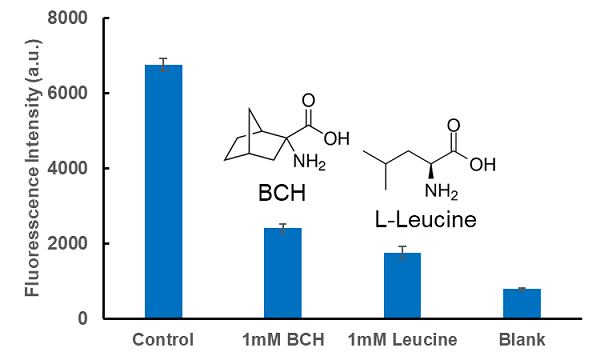
Inhibition of amino acid uptake by LAT1 inhibitor BCH
Cell Line: Human cervical cancer cells, HeLa
Inhibitor: LAT1 Inhibitor, BCH (2-Aminobicyclo[2.2.1]heptane-2-carboxylic acid) and L-Leucine
The intracellular uptake of BPA was inhibited by the addition of BCH, a LAT inhibitor, and L-Leucine, one of the BCAAs.


Inhibition of amino acid uptake by ovalbumin peptide
Provided by Prof. Mamoru Matsubara (Gifu University of Medical Science)
Cell line: Mouse myoblasts, C2C12
The addition of ovalbumin peptide inhibited the uptake of amino acids into C2C12 cells.

3. Cystine uptake and intracellular metabolism
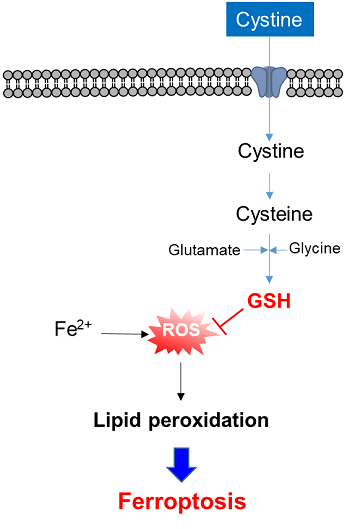
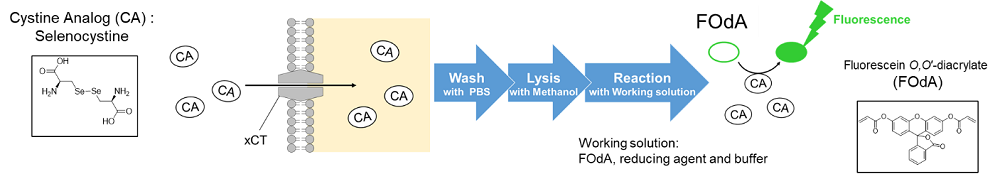
3-1. Cystine uptake assay
Radiolabeled cystine has been the only method to measure xCT activity for many years. However, the radioisotope makes the procedure inconvenient and limits its usage. The Cystine Uptake Assay Kit can measure xCT activity by fluorescence, which is a much simpler approach compared with the radiolabeled method. The Cystine Analog (CA ) contained in the kit is taken up into cells via xCT as well as cystine, and the xCT activity can be measured by the fluorescence detection of the CA.
|
Category |
Product Name (Click the link in the table to view the details) |
|---|---|
|
Cystine Uptake |
3-2. Application: Cystine uptake
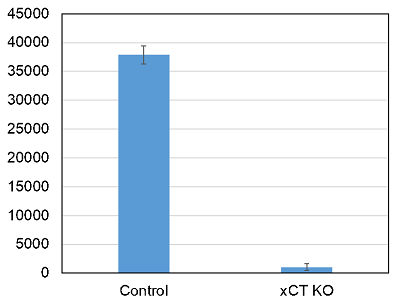
Cystine uptake in wild type and xCT knockdown cells
Provided by Assistant Prof. Hironori Kato (Kyoto University, Graduate School of Biostudies)
Cell line: Human astrocytoma cells, U251
In xCT knockdown cells, almost no cystine uptake was observed.
Inhibition of cystine uptake by sulfasalazine
Provided by Prof. Hideyuki Saya (Keio University, Institute for Advanced Biosciences)
Cell line: Human lung cancer cells, A549; human lung cancer cells, HCT116
Reagents: Cystine transporter inhibitor, sulfasalazine
Cystine uptake was inhibited by the addition of an xCT inhibitor, sulfasalazine (SSZ).

Comparison with radiolabeling: cystine uptake in xCT knockout and overexpressing cells
Provided by Prof. Hideo Sato (Niigata University, School of Health Sciences)
Cell line: Human sarcoma cells, HT1080
The following data showed that the cystine uptake assay is correlated with the radiolabeled cystine method.

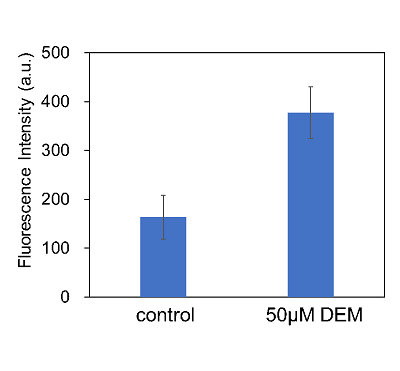
Enhancement of cystine uptake by diethyl maleate
Cell line: Human liver cancer cells, HepG2
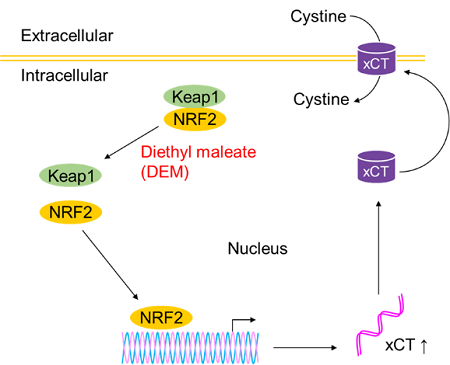
The addition of diethyl maleate(DEM) enhanced cystine uptake by HepG2 cells. The effects of DEM treatment may be because KEAP1 induced xCT expression through the KEAP1–NRF2 pathway.
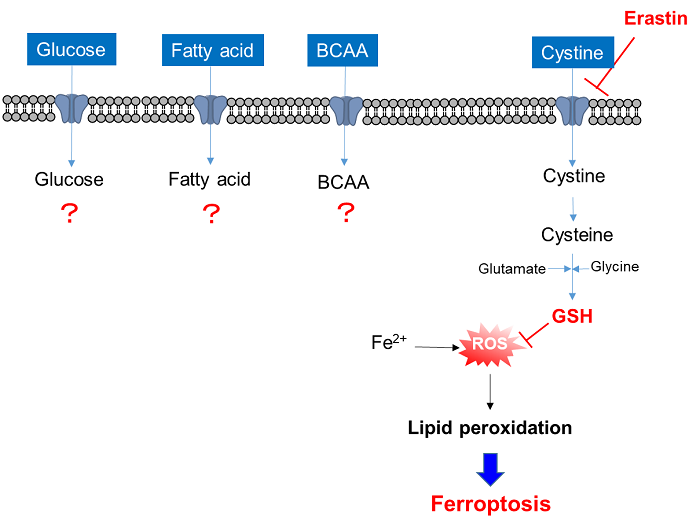
4. Comprehensive evaluation of nutrition uptake
Single stimulation can cause multiple changes in cellular functions. Nutrition uptake is one of these, and comprehensive analysis of the changes in nutrition uptake is important for understanding the cellular functions related to nutrition metabolism.
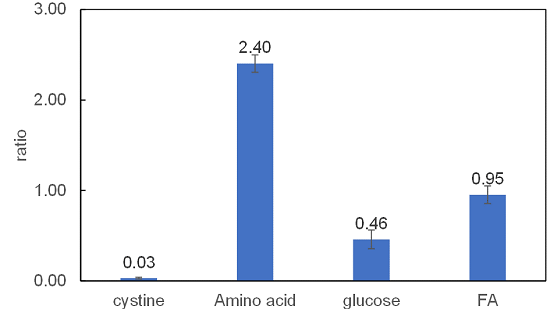
Analysis of changes in nutrition uptake by xCT inhibitor Erastin
Cell line: Human lung cancer cells, A549
Reagent: Cystine transporter inhibitor, erastin
The xCT inhibitor erastin inhibited the uptake of cystine. Furthermore, glucose uptake was also decreased and amino acid uptake was greatly increased. Fatty acid uptake was almost unchanged. Although the mechanism of this phenomenon is unclear, it is possible that erastin shifted the nutrient metabolism from glucose metabolism to amino acid metabolism.