Classification
Chromogen/Metal Indicator
introduction
Metal indicators, colorimetric metal chelators, and fluorometric metal chelators are chromogenic chelating agents. They can be used to determine the metal ion concentration in solutions. There are several methods for measuring a particular metal ion in solution, including chelate titration, colorimetric detection, fluorometric detection, colorimetric detection coupled with solvent extraction, and precipitation titration. In general, metal indicators are utilized for chelate titration, and colorimetric and fluorometric chelating agents are utilized for the determination of the metal concentration by spectrophotometry.

Chelate Titration
Metal indicators and chelating agents are used to determine the concentration of specific metal ions in solution by chelate titration. The endpoint of the titration can be determined by the color of the solution. The color of the metal indicator varies sharply with the association and dissociation of metal ions, so the total amount of a metal ion can be estimated by the amount of titration reagent used. EDTA-metal complexes are very stable and have high dissociation constants. EDTA forms a 1:1 complex with most metal ions that are divalent or more. For these reasons, EDTA is a widely used titration reagent. Fluorescent metal indicators are useful for determining the endpoint of titration of metal ions in stained samples.
Spectrophotometry
Colorimetric chelating reagents form colored complexes with metal ions in pH-controlled solutions. Their selectivity depends on the dissociation constants of metal ions and their sensitivity depends on the molar absorptivity of the complex. However, few colorimetric chelating reagents are highly selective. To increase selectivity, the choice of masking reagents or solvents for the extraction procedures is important. The maximum wavelength of the complex is also an important factor for selectivity and sensitivity. For example, Nitroso-PSAP forms complexes with several heavy metal ions, but the maximum wavelength of the Nitroso-PSAP-Fe complex is considerably longer than that of the other metal complexes. Thus, iron can be determined without interference from other metal ions. Water-soluble colorimetric chelating agents enable the determination of metal ions in aqueous solution without solvent extraction. Therefore, these reagents are useful for automatic detection systems. Calcium is one of the most important metal ions for signal transduction in living cells. Several unique reagents are available for monitoring the calcium concentration in living cells.
Masking Reagent
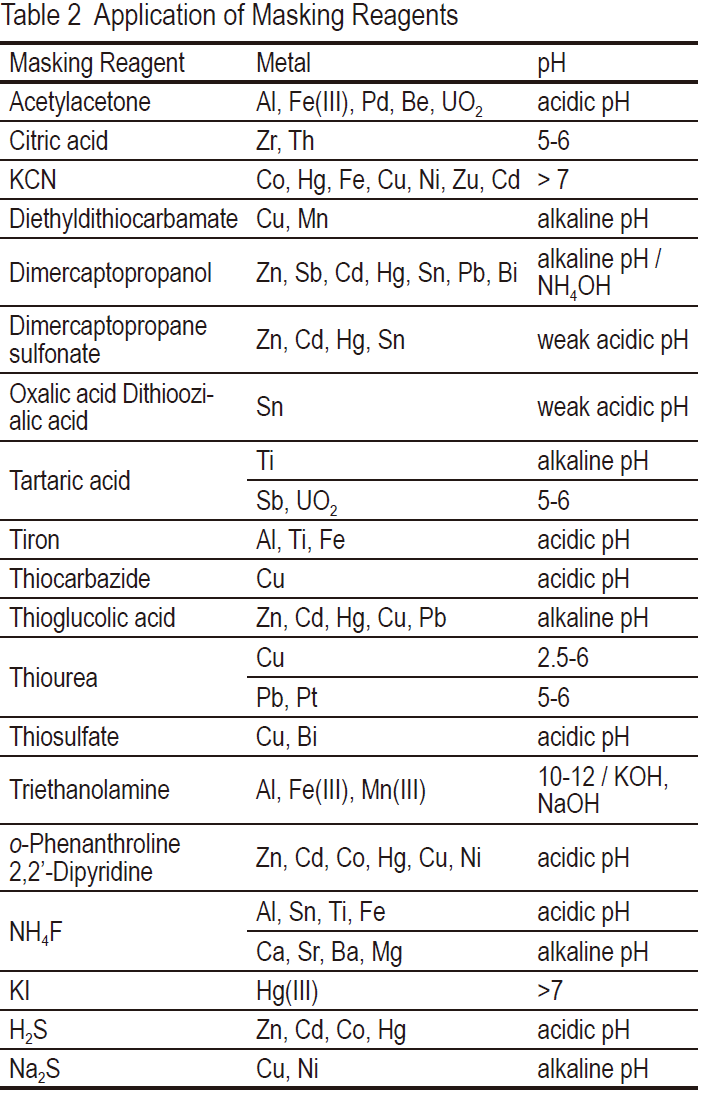
EDTA forms stable complexes with various metal ions in chelate titration. The total consumption of EDTA solution indicates the total amount of mixed metal ions contained in the sample solution. To determine the amount of one specific metal ion in the sample solution, masking reagents should be added to remove other metal ions.Table 2 shows popular masking reagents for chelatometry and colorimetry.
Reagents for Ion Selective Electrodes
Real-time monitoring of electrolytes is increasing in importance for clinical use. For example, monitoring sodium and potassium ion concentrations in the blood flow is indispensable during cardiac surgery. Although lithium ion is used in the treatment of manic symptoms, its serum level must be strictly monitored due to its toxicity. For simple and quick determination of these alkaline and alkaline earth metal ions, polyvinyl chloride (PVC) membrane electrodes have been widely used. The concentration of neutral carriers, plasticizers, and counteranions used to prepare the PVC electrode determines its ion selectivity. A large number of crown ether compounds have been developed. Some of them are superior to naturally existing neutral carriers such as valinomycin, which is highly selective for potassium ions.

Product
- Chromogen/Metal Indicator
-
Code Product name Unit size A006 Metal Indicator
ALC100 mg
1 gA012 Metal Indicator
Arsemate5 g
25 gA015 Metal Indicator
Azomethine H5 g
25 gB002 Metal Indicator
Bathocuproinedisulfonic acid, disodium salt1 g B004 Metal Indicator
Bathophenanthrolinedisulfonic acid, disodium salt1 g B015 Metal Indicator
BT25 g B026 Metal Indicator
5-Br-PAPS100 mg B037 Metal Indicator
Sodium bicinchoninate5 g C001 Metal Indicator
Calcein1 g
5 gC002 Metal Indicator
Calcein Blue1 g C010 Metal Indicator
Chlorophosphonazo-III100 mg
1 gC016 Metal Indicator
Cu-PAN10 g C017 Metal Indicator
Cyanoline Blue25 g D008 Metal Indicator
Diantipyrylmethane25 g D027 Metal Indicator
DAN1 g H007 Metal Indicator
HNB1 g M011 Metal Indicator
Murexide5 g
25 gM012 Metal Indicator
MX100 g N010 Metal Indicator
Nitroso-PSAP100 mg
1 gN012 Metal Indicator
NN diluted with potassium sulfate25 g N013 Metal Indicator
NN1 g N031 Metal Indicator
Nitro-PAPS100 mg P002 Metal Indicator
PAN1 g P003 Metal Indicator
PAR1 g
5 gP004 Metal Indicator
PC1 g
5 gP007 Metal Indicator
o-Phenanthroline5 g
25 gP012 Metal Indicator
PR1 g S003 Metal Indicator
SATP1 g
5 gT003 Metal Indicator
TPPS100 mg X001 Metal Indicator
XB-I1 g
5 gX003 Metal Indicator
XO1 g
5 gZ002 Metal Indicator
Zincon1 g
5 g




