Science Note: Exosome / Endocytosis / Phagocytosis
Endosomal Proteins Regulating Cellular Fate [Sep. 3, 2024]
|
Endosomal proteins play a critical role in determining cell fate by regulating the trafficking and signaling of receptors and other membrane-bound molecules within cells. These proteins control the endocytosis, recycling, or degradation of signaling receptors, thereby influencing key pathways such as those governing differentiation, survival, and apoptosis. By modulating the duration and intensity of signaling cascades, endosomal proteins help determine whether a cell will proliferate, differentiate, or undergo programmed cell death. Dysregulation of these processes can lead to inappropriate cell fate decisions and contribute to diseases such as cancer and neurodegeneration. |
||
|
Ku70 senses cytosolic DNA and assembles a tumor-suppressive signalosome |
MYCT1 controls environmental sensing in human haematopoietic stem cells |
EGFR-dependent endocytosis of Wnt9a and Fzd9b promotes β-catenin signaling during hematopoietic stem cell development in zebrafish |
|
Point of Interest - Ku70 forms a complex with GTPase Ras and kinase Raf at Rab5+Rab7+ early-late endosomes, activating MEK-ERK pathways that reduce cell proliferation and tumorigenesis. - Targeting this Ku70 signalosome could improve cancer treatment by restoring proper DNA repair and cell cycle regulation. |
Point of Interest - MYCT1 is a key regulator of human hematopoietic stem cell (HSC) self-renewal and expansion by modulating endocytosis and environmental sensing. - MYCT1 expression is essential for HSC expansion and engraftment, and its loss leads to excessive endocytosis and dysregulated signaling. - Restoring MYCT1 in cultured HSCs preserves stemness and improves expansion, highlighting its importance in human HSC regulation. |
Point of Interest - EGFR and EPS15-dependent Wnt9a-Fzd9b signaling is essential for hematopoietic stem cell development in zebrafish. - EGFR-mediated phosphorylation triggers endocytosis of Wnt9a-Fzd9b complexes, which are necessary for hematopoietic stem cell signaling. - EPS15 promotes Wnt9a-Fzd9b endocytosis, which is critical for hematopoietic stem cell development in zebrafish and humans. |
| Related Techniques | ||
| Endocytosis Detection detection | ECGreen-Endocytosis Detection | |
| Lysosomal function | Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red | |
| Plasma Membrane Staining | PlasMem Bright Green / Red | |
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | |
| Mitochondrial membrane potential detection | JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit | |
| First-time autophagy research | Autophagic Flux Assay Kit | |
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |
| Cell Proliferation / Cytotoxicity Assay | Cell Counting Kit-8, Cytotoxicity LDH Assay Kit-WST | |
| Ready-to-use kit for Cell cycle assay | Cell Cycle Assay Solution Blue / Deep Red | |
| Related Applications | ||
Phagocytosis assay of labeled apoptotic cells in THP-1 cells
|
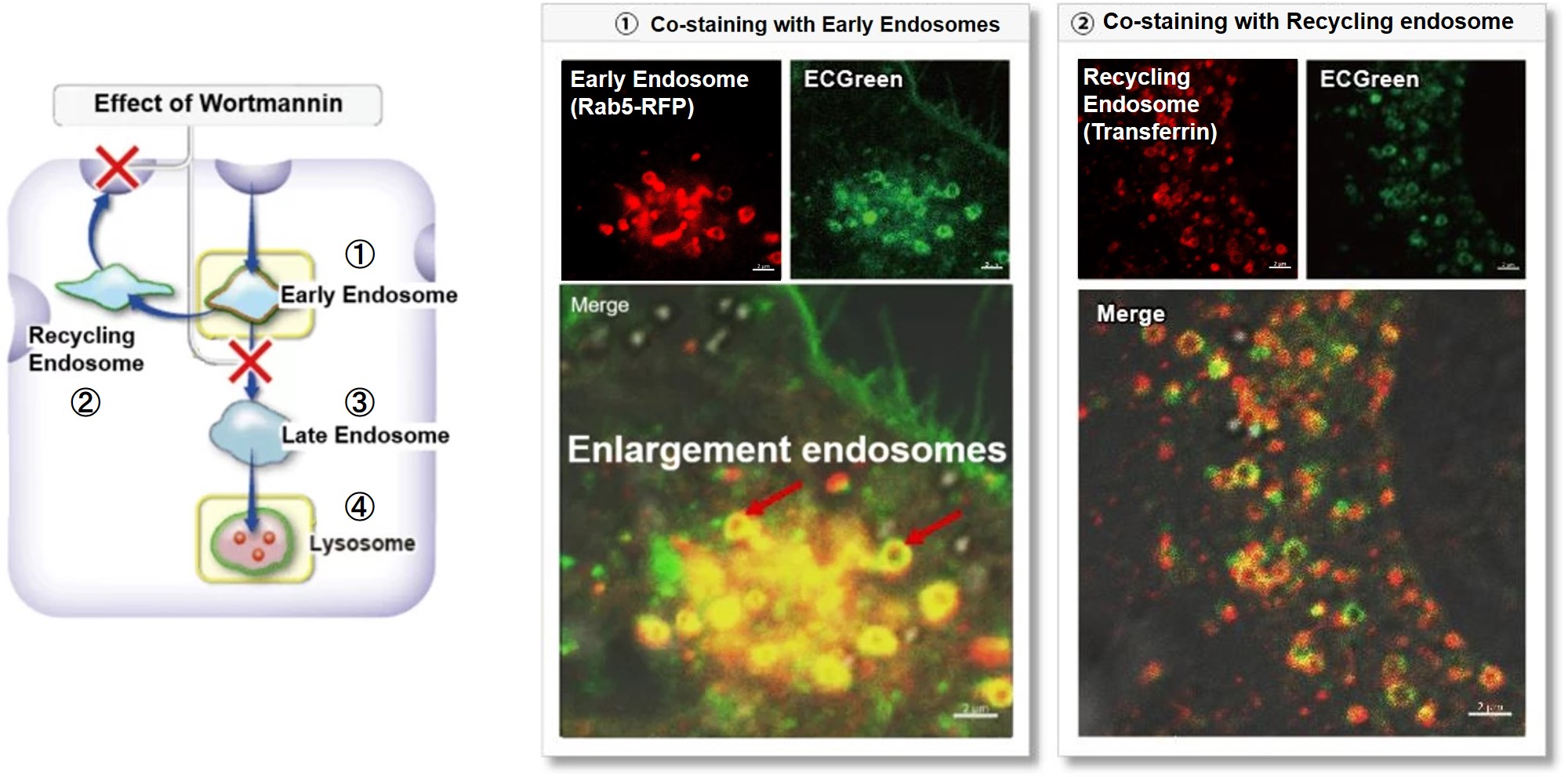
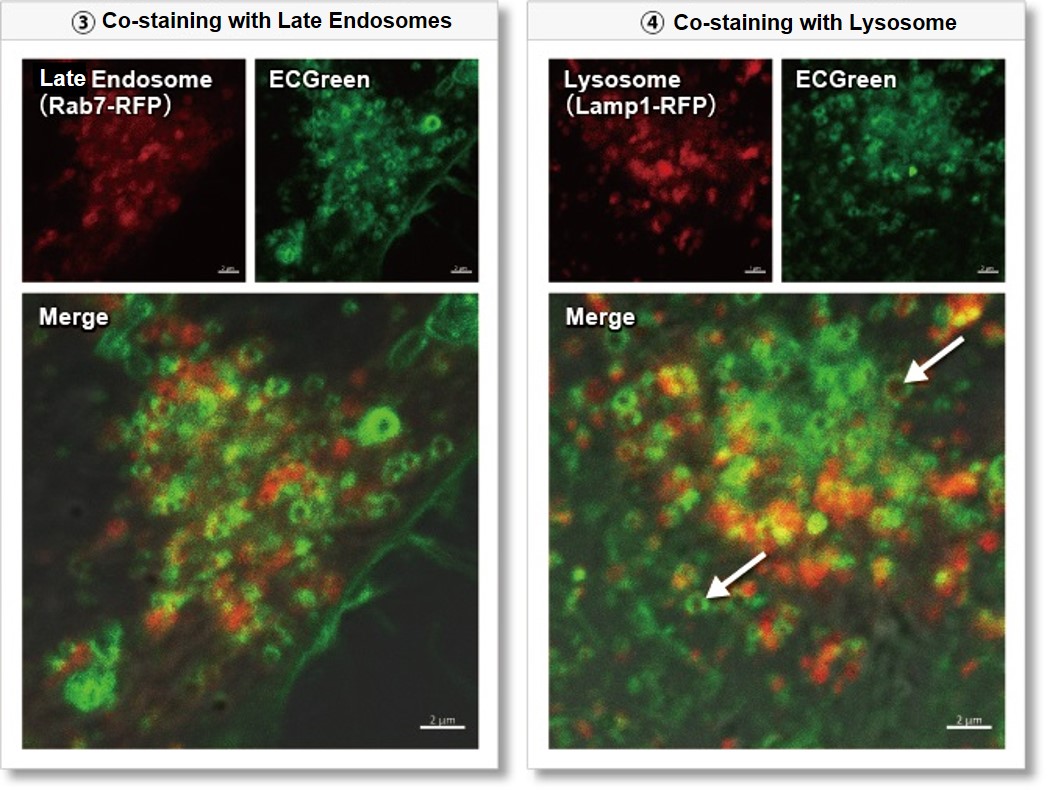
Wortmannin is known to inhibit endosomal recycling and lysosomal translocation, leading to endosomal enlargement. ①Eary endosome: Rab5-RFP (red) As a result, it was confirmed that ECGreen (green) co-localizes only with enlarged early endosomes and recycling endosomes (Fig. ① and ②), but not with late endosomes or Lysosomes (Fig. ③ and ④), supporting Wortmannin's effect. ECGreen can visualize changes in the intracellular vesicular trafficking system and endosome shape. Endosomes (ECGreen, green): Ex. 405 nm / Em. 500 – 560 nm [Experimental Procedure] |
|
Controlling the immune cell helps treat cancer and neurodegenerative disease [Jul. 23, 2024]
Open / Close the Article
|
Immune cell senescence is a critical factor in the progression of several diseases. As immune cells age and enter a senescent state, their ability to respond to infection, eliminate senescent cells, and regulate inflammation diminishes. This impaired immune function may contribute to the development and progression of age-related diseases, including cancer, cardiovascular disease, and neurodegenerative disorders. In addition, the accumulation of senescent immune cells in tissues can create a pro-inflammatory environment that further exacerbates disease pathology and impairs tissue function. |
||
|
Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment |
Senescent alveolar macrophages promote early-stage lung tumorigenesis |
Rejuvenating aged microglia by p16ink4a-siRNA-loaded nanoparticles increases amyloid-β clearance in animal models of Alzheimer’s disease |
|
Point of Interest - Elimination of senescent cells restores CD8 T cell proliferation and reduces immunotherapy resistance. - Anti-senescent cell drugs prior to immune checkpoint inhibitors may enhance the effectiveness of cancer immunotherapy. |
Point of Interest - Senescent alveolar macrophages accumulate early in lung cancer neoplasia and suppress cytotoxic T-cell responses. - Removal of these macrophages reduces adenoma development, indicating their role in promoting tumorigenesis. - Targeting senescent macrophages may slow lung cancer progression by altering the local microenvironment. |
Point of Interest - Reduced amyloid clearance in Alzheimer's disease is associated with decreased phagocytic ability of older microglia. - Downregulation of p16ink4a, a cell cycle factor associated with microglial aging, by siRNA increases amyloid clearance by phagocytosis and reverses cognitive deficits in Alzheimer's disease mode. - Targeting p16ink4a with nanoparticles to rejuvenate microglia offers a potential treatment strategy for Alzheimer's disease. |
| Related Techniques | ||
| Phagocytosis Assay | AcidSensor Labeling Kit – Endocytic Internalization Assay and Cellstain- Calcein-AM solution | |
| Endocytosis Detection detection | ECGreen-Endocytosis Detection | |
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples. | |
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |
| Cell Proliferation / Cytotoxicity Assay | Cell Counting Kit-8, Cytotoxicity LDH Assay Kit-WST | |
| Ready-to-use kit for Cell cycle assay | Cell Cycle Assay Solution Blue / Deep Red | |
| Lipid Droplet detection | Lipid Droplet Assay Kit - Blue / Deep Red | |
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | |
| Related Applications | ||
Phagocytosis assay of labeled apoptotic cells in THP-1 cells
|
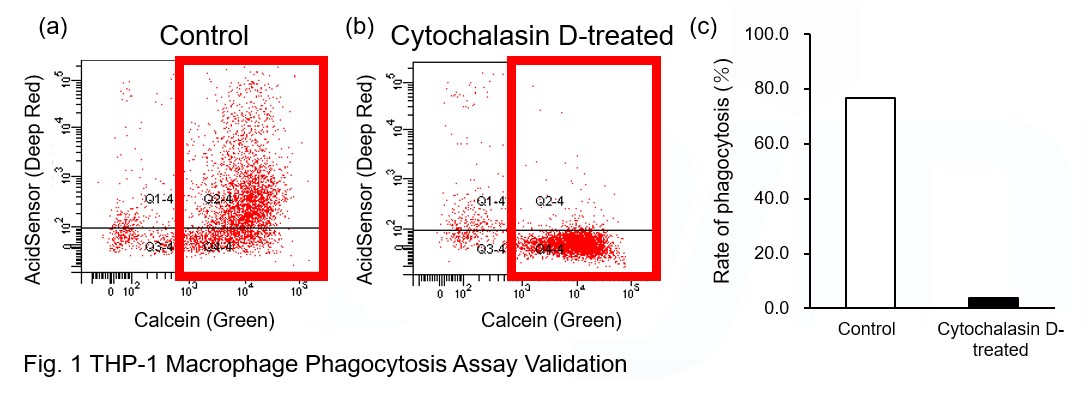
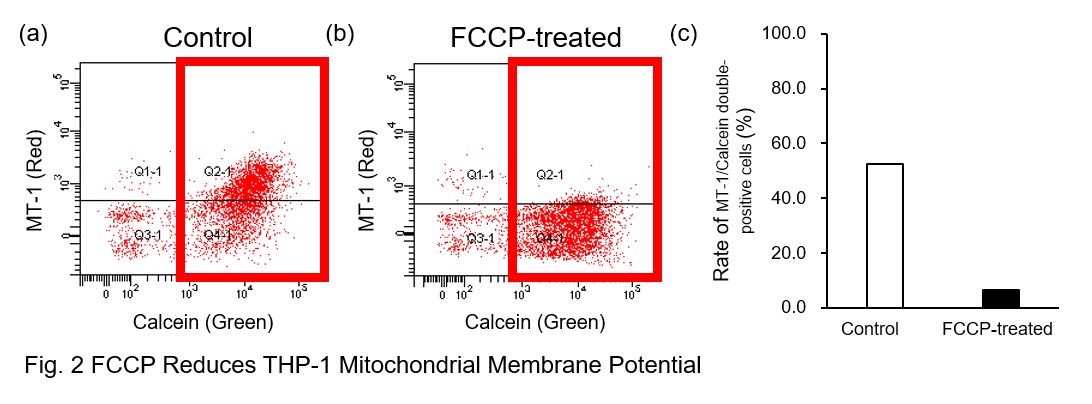
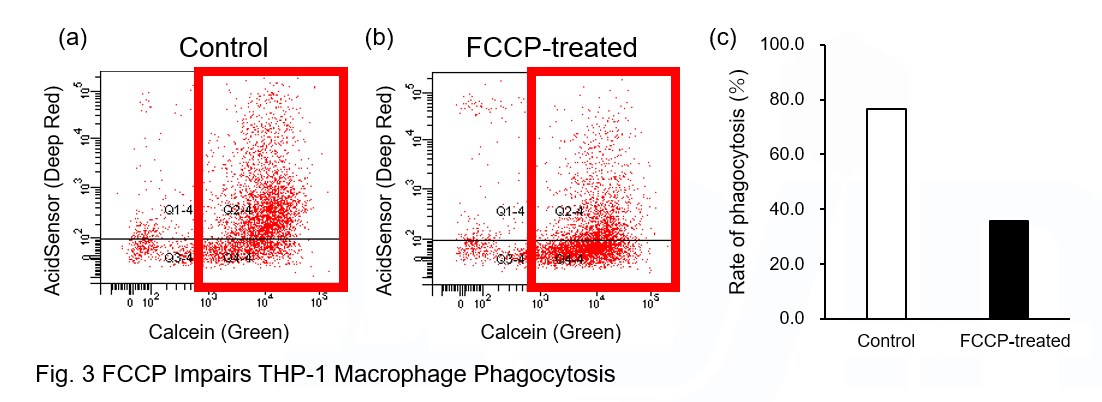
AcidSensor-labeled substances are taken up by cells and their fluorescence increases when they reach acidic organelles such as lysosomes. Taking advantage of this property, we evaluate the phagocytic activity of apoptotic cells by co-culturing AcidSensor-labeled apoptotic cells with Calcein-labeled THP-1 macrophages. As a result, Calcein (Green) / AcidSensor (Deep red) double-positive cells, indicating THP-1 macrophages phagocytosing apoptotic cells, were observed by flow cytometry (Fig. 1a). Furthermore, when the phagocytosis of THP-1 macrophages was inhibited by Cytochalasin D, the percentage of double-positive cells decreased (Fig. 1b and 1c), confirming that the assay system can accurately evaluate phagocytosis. A recent report reveals that inhibition of mitochondrial function induces a switch to glycolysis and reduces phagocytosis in cultured microglia, resident macrophages in the central nervous system*. To replicate this result, phagocytosis assays were performed using mitochondria-inhibited THP-1 macrophages. The results show that FCCP, a potent uncoupler of oxidative phosphorylation in mitochondria, decreases mitochondrial membrane potential (MT-1, Red) of THP-1 macrophages (Fig. 2) and reduces phagocytosis (Fig. 3). *Lauren H. Fairley, et al., PNAS (2023)
|
|
Open >> Experimental ProcedurePreparation of AcidSensor-labeled apoptotic cells (day before assay) 1. Add 10 μl of DMSO to NH2-Reactive AcidSensor and dissolve. 5 μl NH2-Reactive AcidSensor solution was added to 5 ml HBSS to make the Working solution (1000-fold dilution).
Phagocytosis assay using THP-1 macrophages 1. To differentiate THP-1 cells into macrophages, THP-1 cells were seeded into 6 well plates at 1x106 cells/well and incubated with 100 nM PMA for 3 days in an incubator. |
||
Metabolic shift to glycolysis in senescenct cells
|
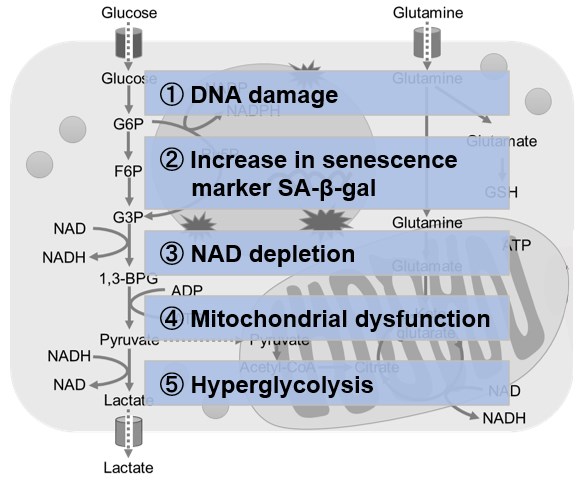
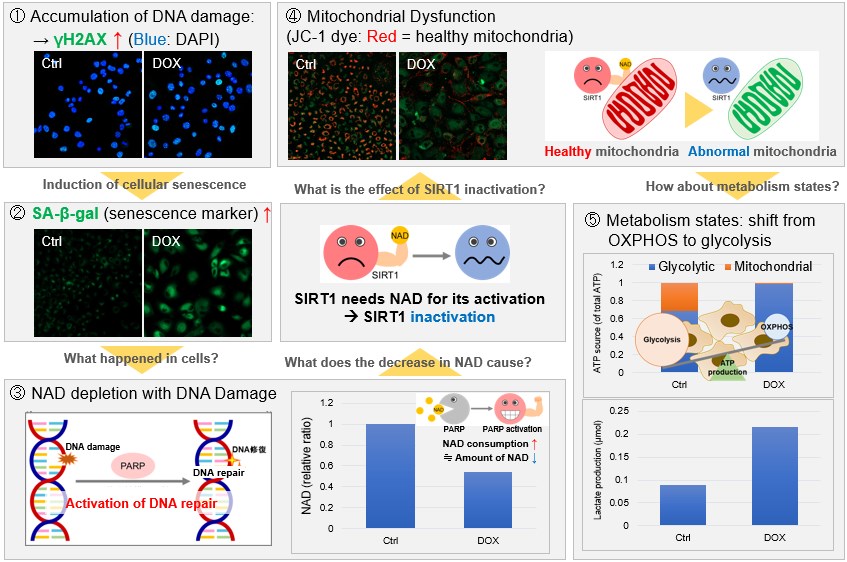
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
|
|
|
||
New function of endocytosis induced and inhibited by membrane proteins [Jun. 26, 2024]
Open / Close the Article
|
Endocytosis induction refers to the initiation of the endocytic process, where specific signals trigger the cell to internalize extracellular substances. This induction often involves membrane proteins that can act as receptors to recognize and bind external ligands, initiating the formation of endocytic vesicles. The interaction between ligands and membrane proteins not only triggers endocytosis, but also ensures that the internalized cargo is selectively processed. Thus, membrane proteins are integral to both the initiation and specificity of endocytosis, influencing cellular uptake and signaling pathways. |
||
|
Endocytic vesicles act as vehicles for glucose uptake in response to growth factor stimulation |
Endocytosis blocks the vesicular secretion of exosome marker proteins |
Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis |
|
Point of Interest - PDGF receptor (PDGFR) co-endocytoses with subset of glucose transporter 1 (GLUT1/SLC2A1) upon PDGF-stimulation. - The PDGFR/GLUT1-containing endosomes have multiple glycolytic enzymes and localize to adjacent mitochondria. - The glucose-loaded endosomes generated by growth factors deliver glucose to the glycolytic machinery in proximity to mitochondria. |
Point of Interest - High expression of CD63, CD81 or CD9 inhibits its own endocytosis and induces its plasma membrane accumulation and vesicular secretion. - Induction of endocytosis inhibits their vesicular secretion and, in the case of CD9 and CD81, causes their destruction in the lysosome. - Vesicular secretion of exosome marker proteins occurs primarily through an endocytosis-independent pathway. |
Point of Interest - Upon aggregation, even canonical clathrin-dependent cargoes are redirected to the aggregation-dependent endocytosis (ADE) pathway. - ADE is an actin-driven process that morphologically resembles macropinocytosis. - ADE clears stress-induced receptor aggregates and facilitates their lysosomal degradation to maintain cell surface proteostasis. |
| Related Techniques | ||
| Endocytosis Detection detection | ECGreen-Endocytosis Detection | |
| Exosome Labeling | ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red | |
| Lysosomal function | Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red | |
| Plasma Membrane Staining | PlasMem Bright Green / Red | |
| Phagocytosis Assay | AcidSensor Labeling Kit – Endocytic Internalization Assay and Cellstain- Calcein-AM solution | |
| Autophagy detection | Autophagic Flux Assay Kit | |
| Mitophagy or autophagy detection | Mitophagy Detection Kit | |
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |
| Related Applications | ||
Clear visualization of intracellular vesicular trafficking
|
Wortmannin is known to inhibit endosomal recycling and lysosomal translocation, leading to endosomal enlargement. ①Eary endosome: Rab5-RFP (red) As a result, it was confirmed that ECGreen (green) co-localizes only with enlarged early endosomes and recycling endosomes (Fig. ① and ②), but not with late endosomes or Lysosomes (Fig. ③ and ④), supporting Wortmannin's effect. ECGreen can visualize changes in the intracellular vesicular trafficking system and endosome shape. Endosomes (ECGreen, green): Ex. 405 nm / Em. 500 – 560 nm [Experimental Procedure] |
|
Phagocytosis assay of labeled apoptotic cells in THP-1 cells
|
AcidSensor-labeled substances are taken up by cells and their fluorescence increases when they reach acidic organelles such as lysosomes. Taking advantage of this property, we evaluate the phagocytic activity of apoptotic cells by co-culturing AcidSensor-labeled apoptotic cells with Calcein-labeled THP-1 macrophages. As a result, Calcein (Green) / AcidSensor (Deep red) double-positive cells, indicating THP-1 macrophages phagocytosing apoptotic cells, were observed by flow cytometry (Fig. 1a). Furthermore, when the phagocytosis of THP-1 macrophages was inhibited by Cytochalasin D, the percentage of double-positive cells decreased (Fig. 1b and 1c), confirming that the assay system can accurately evaluate phagocytosis. A recent report reveals that inhibition of mitochondrial function induces a switch to glycolysis and reduces phagocytosis in cultured microglia, resident macrophages in the central nervous system*. To replicate this result, phagocytosis assays were performed using mitochondria-inhibited THP-1 macrophages. The results show that FCCP, a potent uncoupler of oxidative phosphorylation in mitochondria, decreases mitochondrial membrane potential (MT-1, Red) of THP-1 macrophages (Fig. 2) and reduces phagocytosis (Fig. 3). *Lauren H. Fairley, et al., PNAS (2023)
|
|
|
[Experimental Procedure] Preparation of AcidSensor-labeled apoptotic cells (day before assay) 1. Add 10 μl of DMSO to NH2-Reactive AcidSensor and dissolve. 5 μl NH2-Reactive AcidSensor solution was added to 5 ml HBSS to make the Working solution (1000-fold dilution).
Phagocytosis assay using THP-1 macrophages 1. To differentiate THP-1 cells into macrophages, THP-1 cells were seeded into 6 well plates at 1x106 cells/well and incubated with 100 nM PMA for 3 days in an incubator. |
||
Membrane Trafficking: Organizer of Intra- and Extracellular Homeostasis [Feb. 6, 2024]
Open / Close the Article
|
Membrane trafficking involves vital processes such as phagocytosis, endocytosis, and exocytosis, each of which plays a critical role in cellular homeostasis. Phagocytosis and endocytosis allow cells to ingest external particles and fluids, facilitating nutrient uptake and the removal of pathogens or debris, which is critical for cellular health and immune response. Exocytosis, on the other hand, allows cells to expel waste products and secrete signaling molecules that are essential for communication and functional coordination within tissues. These coordinated processes ensure that the cell maintains a balanced internal environment, adapts to changing external conditions, and maintains cellular integrity and function. |
||
|
CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma |
Stress granules plug and stabilize damaged endolysosomal membranes |
A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion |
|
Point of Interest - IFNγ, derived from Th1 cells, stimulates the induction of MHC-II+ microglia, which sustains Th1-mediated anti-glioma immunity. - Th1 cells enhance microglial phagocytosis of gliomas through AXL-MER receptor signaling. - The presence of microglia and MHC-II expression are associated with protective outcomes in human glioblastoma. |
Point of Interest - This process requires both ESCRT (endosomal sorting complex required for transport)-dependent and independent mechanisms. - Blocking stress granule formation in human macrophages creates a permissive environment for Mycobacterium tuberculosis, which exploits endomembrane damage to survive in the host. |
Point of Interest - Phosphatidylinositol 4-kinase type IIα on the surface of MVEs mediates the recruitment of the exocyst complex. - The exocyst targets MVEs to the plasma membrane for exosome secretion. - Disruption of PI(4)P generation or exocyst function blocked exosomal secretion of programmed death ligand 1 (PD-L1), a key immune checkpoint protein in tumor cells, and led to its accumulation in lysosomes. |
| Related Techniques | ||
| Phagocytosis Assay | AcidSensor Labeling Kit – Endocytic Internalization Assay and Cellstain- Calcein-AM solution | |
| Endocytosis Detection detection | ECGreen-Endocytosis Detection | |
| Exosome Labeling | ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red | |
| Plasma Membrane Staining | PlasMem Bright Green / Red | |
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | |
| Antibody/Protein labeling with fast and high recovery |
Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2 | |
| Related Applications | ||
Phagocytosis assay of labeled apoptotic cells in THP-1 cells
|
AcidSensor-labeled substances are taken up by cells and their fluorescence increases when they reach acidic organelles such as lysosomes. Taking advantage of this property, we evaluate the phagocytic activity of apoptotic cells by co-culturing AcidSensor-labeled apoptotic cells with Calcein-labeled THP-1 macrophages. As a result, Calcein (Green) / AcidSensor (Deep red) double-positive cells, indicating THP-1 macrophages phagocytosing apoptotic cells, were observed by flow cytometry (Fig. 1a). Furthermore, when the phagocytosis of THP-1 macrophages was inhibited by Cytochalasin D, the percentage of double-positive cells decreased (Fig. 1b and 1c), confirming that the assay system can accurately evaluate phagocytosis. A recent report reveals that inhibition of mitochondrial function induces a switch to glycolysis and reduces phagocytosis in cultured microglia, resident macrophages in the central nervous system*. To replicate this result, phagocytosis assays were performed using mitochondria-inhibited THP-1 macrophages. The results show that FCCP, a potent uncoupler of oxidative phosphorylation in mitochondria, decreases mitochondrial membrane potential (MT-1, Red) of THP-1 macrophages (Fig. 2) and reduces phagocytosis (Fig. 3). *Lauren H. Fairley, et al., PNAS (2023)
|
|
|
[Experimental Procedure] Preparation of AcidSensor-labeled apoptotic cells (day before assay) 1. Add 10 μl of DMSO to NH2-Reactive AcidSensor and dissolve. 5 μl NH2-Reactive AcidSensor solution was added to 5 ml HBSS to make the Working solution (1000-fold dilution).
Phagocytosis assay using THP-1 macrophages 1. To differentiate THP-1 cells into macrophages, THP-1 cells were seeded into 6 well plates at 1x106 cells/well and incubated with 100 nM PMA for 3 days in an incubator. |
||
TREM2 directs microglial amyloid-beta response [Dec. 12, 2023]
Open / Close the Article
|
Scientists have discovered that Microglia respond to amyloid-beta through the activation of signaling mechanisms mediated by TREM2, involving Syk and DAP10. In mice expressing the Alzheimer’s disease-associated human TREM2R47H allele, antibody-mediated activation of Syk rescues microglial activation. |
|||
|
TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways |
|||
|
Point of Interest |
|||
|
Related Techniques |
|||
| Phagocytosis Assay | AcidSensor Labeling Kit – Endocytic Internalization Assay | ||
| Endocytosis detection | ECGreen-Endocytosis Detection | ||
| Lipid droplet detection | Lipi-Blue / Green / Red / Deep Red | ||
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) |
||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
|
Related Applications |
|||
|
Phagocytosis assay of labeled apoptotic cells in THP-1 cells |
|||
|
|||
|
[Experimental Procedure] Preparation of AcidSensor-labeled apoptotic cells (day before assay)
Phagocytosis assay using THP-1 macrophages
|
|||
Microglial Phagocytosis under mitochondrial Control [Nov. 28, 2023]
Open / Close the Article
|
Scientists have discovered that the mitochondrial translocator protein (TSPO) and hexokinase-2 play key roles in controlling microglial metabolism and phagocytosis. Microglia lacking TSPO resembled dysfunctional microglia observed in aging and Alzheimer's disease, and this could be partially reversed by blocking hexokinase-2 binding to mitochondria. They conclude that targeting mitochondrial hexokinase-2 binding may provide an immunotherapeutic approach to inhibit glycolytic metabolic reprogramming and promote microglial phagocytosis in Alzheimer's disease. |
|||
|
Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease |
|||
|
Point of Interest |
|||
|
Related Techniques |
|||
| Phagocytosis Assay | AcidSensor Labeling Kit – Endocytic Internalization Assay | ||
| Endocytosis detection | ECGreen-Endocytosis Detection | ||
| Lipid droplet detection | Lipi-Blue / Green / Red / Deep Red | ||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | ||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
|
Related Applications |
|||
|
Phagocytosis assay of labeled apoptotic cells in THP-1 cells |
|||
|
|||
|
[Experimental Procedure] Preparation of AcidSensor-labeled apoptotic cells (day before assay)
Phagocytosis assay using THP-1 macrophages
|
|||
Exocytic/Endocytic Pathways in Cancer and Lipid Metabolism [Aug. 22, 2023]
Open / Close the Article
| In recent years, significant advancements have been made in the understanding of novel exo/endocytic pathways in metabolism. These discoveries have garnered considerable attention, particularly in the field of oncology. For instance, tumor-derived extracellular vesicles have been identified as critical mediators in cancer-induced hepatic reprogramming. Their role, along with TNF inhibition, offers a targetable pathway for both preventing fatty liver formation and enhancing the efficacy of chemotherapy. Another groundbreaking observation reveals that the ingestion of tumor-derived microparticles by macrophages induces a rapid metabolic and phenotypic switch. This change subsequently results in decreased motility in the early stages of metastasis in the lung. Additionally, research has demonstrated that phospholipase D6, a protein found on the mitochondrial outer membrane, accelerates the transport of LDL-LDLR from endocytic vesicles to mitochondria, thereby supporting steroidogenesis. | |||
|---|---|---|---|
|
Tumour extracellular vesicles and particles induce liver metabolic dysfunction Click here for the original article: Gang Wang, et al., Nature, 2023 |
Uptake of tumor-derived microparticles induces metabolic reprogramming of macrophages in the early metastatic lung Click here for the original article: Kelly Kersten, et al., Cell Reports, 2023 |
Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis Click here for the original article: Yu-Xia Zhou, et al., Nature Cell Biology, 2023 |
|
|
- All subpopulations of tumor-derived extracellular vesicles and particles (EVPs) could dysregulate liver function. - The fatty acid cargo of tumor EVPs induced secretion of tumor necrosis factor (TNF) by Kupffer cells, promoting fatty liver formation. - Kupffer cell ablation or TNF blockade significantly reduced tumor-induced fatty liver formation. |
- Ingestion of tumor-derived material leads to the phenotypic reprogramming of macrophages. - The reprogramming of macrophages influences their patrolling behavior in response to tumor cells. - ZsGreen+ macrophages demonstrate elevated mitochondrial metabolism, characterized by oxidative phosphorylation (OXPHOS). - mTORC1 is essential for enhanced oxidative phosphorylation (OXPHOS) and ATP production in ZsGreen+ macrophages. |
- PLD6 promotes the entrance of LDL and LDLR into the mitochondria. - The fusogenic lipid phosphatidic acid generated by PLD6 facilitates the membrane fusion of LDLR vesicles with the mitochondria. - This intracellular transport pathway of LDL–LDLR bypasses the lysosomes and delivers cholesterol to the mitochondria for steroidogenesis. |
|
| Related Technique in This Topic | |||
| Endocytosis detection | ECGreen-Endocytosis Detection | ||
| pH sensor labeling kit | AcidSensor Labeling Kit – Endocytic Internalization Assay | ||
| Exosome membrane labeling kit | ExoSparkler Exosome Membrane Labeling Kit-Green / Red / Deep Red | ||
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | ||
| Fatty acid uptake assay | Fatty Acid Uptake Assay Kit | ||
| Plasma membrane staining | PlasMem Bright Green / Red | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Related Applications | |||
|
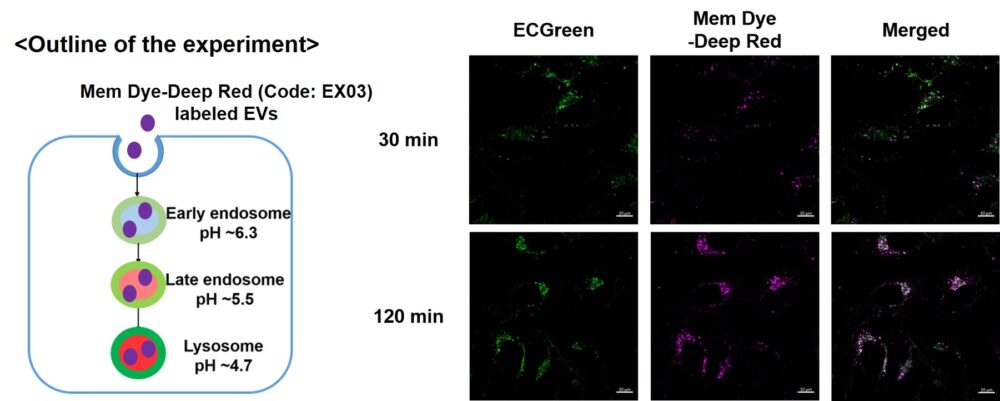
Visualization of EVs uptake via endocytic pathway
|
Mem Dye-labeled EVs are internalized via endocytosis: HeLa cells were incubated with 10 μmol/L ECGreen for 30 min. Then, Mem Dye-Deep Red labeled EVs (quantified as 10 µg of protein) were added to HeLa cells. After 30 or 120 min incubation, the cells were washed and observed under a fluorescence microscope (Scale Bar: 10 µm). |
||
|
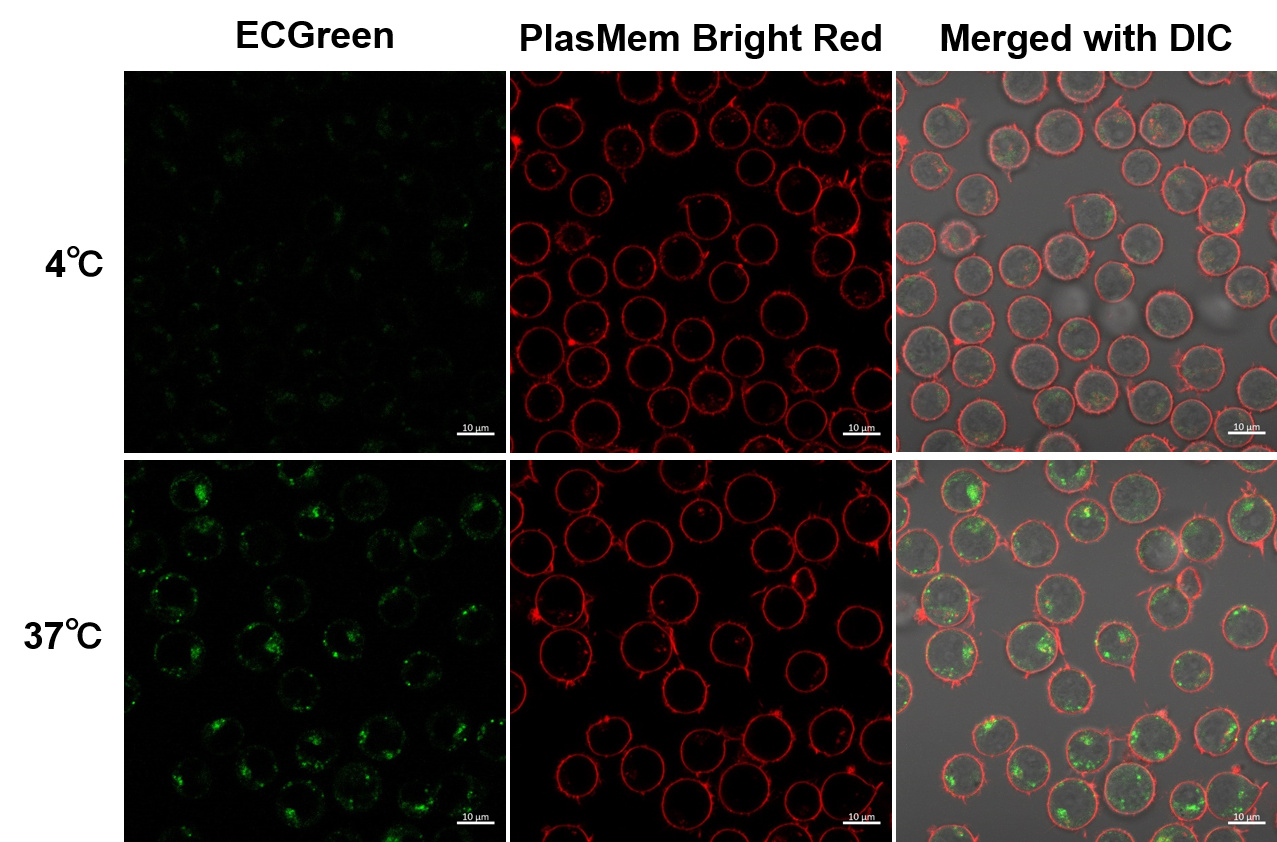
Observation of temperature-dependent endocytosis changes in floating cells
|
Temperature-dependent changes in endocytosis of Jurkat cells were visualized using ECGreen-Endocytosis Detection and PlasMem Bright Red. Cold incubation inhibits the endocytic pathway as observed with ECGreen and PlasMem Bright red. | ||
Mitochondrial Protein VDAC2 Links PI3K to Endosome Maturation [Jun. 13, 2023]
Open / Close the Article
| Scientists have unveiled that VDAC2, a mitochondrial pore protein, as a PI3K-binding protein and show that endosomes positive for Ras PI3K complexes are tethered to mitochondria through PI3K-VDAC2 interaction. These researchers have further elucidated that this mitochondrion-endosome association fosters endosome maturation. Learn more about how the authors detected this mitochondria-endosome interaction using ECGreen for endosome labeling, and PlasMem Bright Red for plasma membrane labeling, which served as a negative control (please refer to Figures 5C - E and 5F - G). | |
|
Interaction between PI3K and the VDAC2 channel tethers Ras-PI3K-positive endosomes to mitochondria and promotes endosome maturation Point of Interest |
|
| Related Techniques | |
| Glycolytic/Mitochondrial activity detection | Glycolysis/JC-1 MitoMP Assay Kit NEW |
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit HOT |
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection |
| Endocytosis detection | ECGreen |
| pH Sensor Labeling kit | AcidSensor Labeling Kit – Endocytic Internalization Assay |
| Cell membrane staining | PlasMem Bright Green / Red |
| Related Applications | |
Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism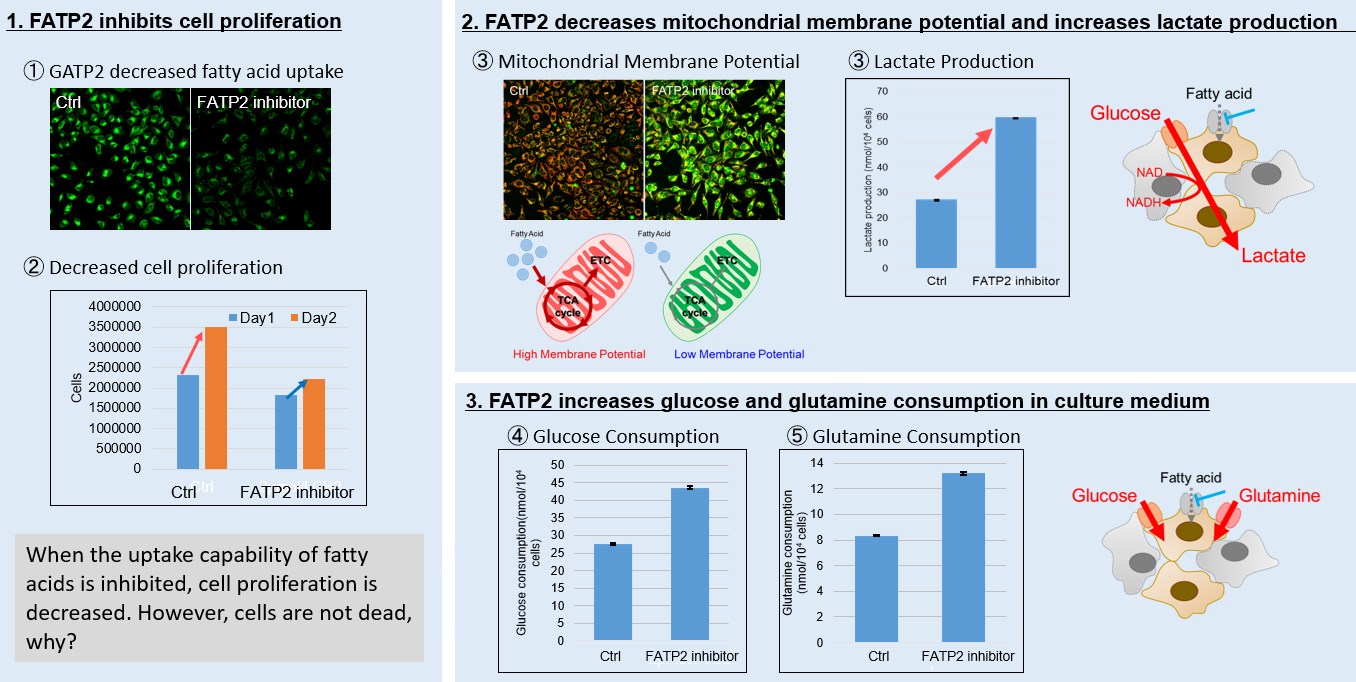
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells: ・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1). ・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2) ・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3) Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay and Mitochondrial Membrane Potential Detection
|
|
Extracellular vesicle-mediated communications in disease [Jan. 24, 2023]
Open / Close the Article
| Exosomes, a form of extracellular vesicles (EVs), mediate intercellular communication by exchanging proteins, DNA, RNA, and lipids between donor and recipient cells, and modulate the biological activities in target cells through receptor-ligand interactions. Recent findings suggest that EV-mediated communications contribute to malignant transformation and the metastasis of cancer, and several disease progressions. Consequently, EVs are attracting considerable interest in the scientific community. Today, we introduce you to three highlighted articles focusing on EVs-mediated communications related to diabetes, immunosystem, and aging. | ||
|---|---|---|
| Communication through EVs between organs in diabetes | Immumoprotective effect of EVs in T cells | Young EVs treatment can promote healthy aging |
| Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance (Jin Wang, et al., Cell Metabolism, 34, 1264-1279, 2022) |
An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory (Alessio Lanna, et al., Nature Cell Biology, 24, 1461-1474, 2022) |
Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice (Jorge Sanz-Ros, et al., Science Advances, 8, eabq22, 2022) |
|
|
|
| Related Technique in This Topic | ||
| EVs labeling | ExoSparkler Exosome Membrane Labeling Kit-Green, Red, Deep Red | |
| EVs Isolation | ExoIsolator Exosome Isolation Kit HOT | |
| Endocytosis detection | ECGreen | |
| Endocytic internalization assay | AcidSensor Labeling Kit HOT | |
| Lysosomal function assay | Lysosomal pH and mass detection Kit HOT | |
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |
| Cellular senescence detection | SPiDER-βGal | |
| Glucose assay | Glucose Assay Kit-WST | |
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | |
Learn more about application data with multiple products here