Science Note: Autophagy
Autophagy Supports Cellular Health and Extends Lifespan [Nov. 19, 2024]
Open / Close the Article
|
Recent research shows that autophagy inhibits protein aggregation and DNA lesions in cells, leading to cellular health and extended lifespan. Here are some of the papers that show some mechanisms and factors that control autophagy for cellular homeostasis. Autophagy is a cellular process that removes damaged organelles and proteins to maintain cellular homeostasis and function. By reducing the accumulation of toxic aggregates and dysfunctional components, autophagy helps to prevent cellular stress and inflammation, key contributors to aging. Increased autophagic activity is associated with improved proteostasis, stress resistance and increased lifespan in several model organisms. Thus, autophagy is a critical mechanism linking cellular health to healthy aging and longevity. |
||||
| The neurotrophic factor MANF regulates autophagy and lysosome function to promote proteostasis in Caenorhabditis elegans Click here for the original article: Shane K. B. Taylor, et. al., PNAS, 2024. |
C16ORF70/MYTHO promotes healthy aging in C.elegans and prevents cellular senescence in mammals Click here for the original article: Anais Franco-Romero, et. al., J Clin Invest., 2024. |
TEX264 drives selective autophagy of DNA lesions to promote DNA repair and cell survival Click here for the original article: Pauline Lascaux, et. al., Cell, 2024. |
||
|
Point of Interest - MANF-1 localizes to lysosomes, modulates autophagic flux and reduces protein aggregation, thereby promoting neuronal survival and stress response. - MANF is essential for cellular proteostasis, stress response and regulation of ageing through ER-lysosomal localisation and transcriptional signaling. |
Point of Interest - MYTHO deletion shortens lifespan, reduces oxidative stress resistance and disrupts autophagy, highlighting its role in healthy aging. - MYTHO is a transcriptionally regulated autophagy initiator essential for stress resistance and longevity in C. elegans and humans. |
Point of Interest - The autophagy receptor TEX264 detects TOP1cc at replication forks and triggers p97-mediated lysosomal delivery through MRE11- and ATR-dependent pathways. - The conserved role of selective autophagy in DNA repair supports cell survival and is clinically relevant. |
||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit | |||
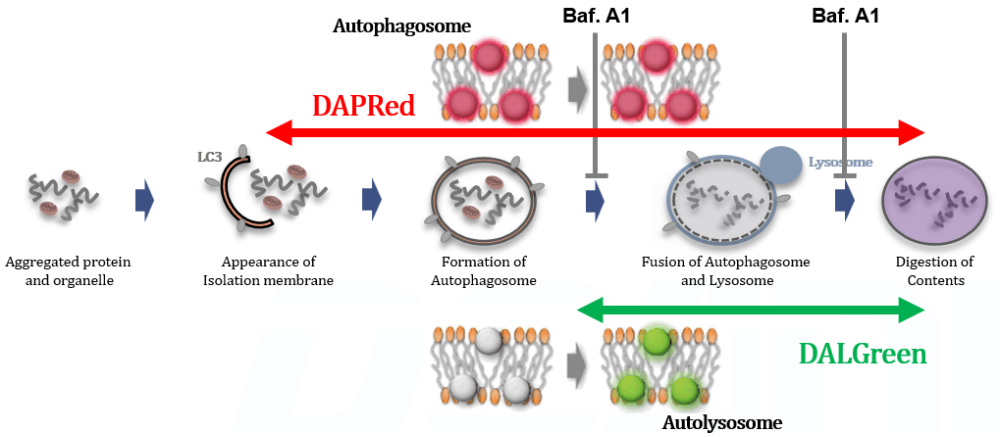
| Autophagy detection | DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples | |||
| NEW SPiDER-βGal Blue for fixed cell and for multiple staining with immunostaining and other methods | ||||
| Mitophagy detection | FerroOrange(intracellular), Mito-FerroGreen(mitochondrial) | |||
| Glutathione Quantification | Mitophagy Detection Kit | |||
| Mitochondrial membrane potential detection | JC-1 MitoMPDetection Kit, MT-1 MitoMPDetection Kit | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit, Extracellular OCR Plate Assay Kit | |||
| Apoptosis detection in multiple samples | NEW Annexin V Apoptosis Plate Assay Kit | |||
| Cell proliferation/ cytotoxicity assay | Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST | |||
| Related Applications | ||||
Analysis of autophagic flux without transfection
Experimental Data
Experimental Conditions Procedure Products in Use
|
||||
The Role of Autophagy in Neuronal Health and Function [Sep. 27, 2024]
Open / Close the Article
|
Autophagy is a cellular process that degrades and recycles damaged cellular components and is critical for maintaining cellular health and function. In neurons, autophagy plays an important protective role by removing defective proteins and organelles, preventing the accumulation of toxic aggregates that can lead to neurodegenerative diseases. This process also helps maintain synaptic plasticity and neuronal homeostasis, which are essential for proper neuronal function and brain health. In addition, by regulating the response to neural stress, autophagy contributes to the overall resilience and longevity of neural cells. |
||||
| The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34 Click here for the original article: Rasmus Berglund, et. al., Nature Communications, 2024. |
Lysophagy protects against propagation of α-synuclein aggregation through ruptured lysosomal vesicles Click here for the original article: Keita Kakuda, et. al., PNAS, 2023. |
The Hippo pathway noncanonically drives autophagy and cell survival in response to energy stress Click here for the original article: Gayoung Seo, et. al., Molecular Cell, 2023. |
||
|
Point of Interest - This population is found to be dependent on IL-34, a ligand for CSF1R. - Loss of autophagy-dependent microglia leads to neuronal and glial cell death and increased mortality in aged mice exposed to neuroinflammation. - Conversely, IL-34-mediated microglial expansion is protective. |
Point of Interest - This rupture seeded the aggregation of endogenous αSyn, initially around damaged lysosomes. - Exogenous αSyn aggregates induced the accumulation of LC3 on lysosomes, indicating the activation of lysophagy. - Autophagy-deficient cells treated with αSyn aggregates had an increased number of ruptured lysosomes and enhanced propagation of αSyn aggregates. |
Point of Interest - MAP4K2 phosphorylates LC3A at Serine 87 to promote autophagic flux. - Energy stress induces the formation of the MAP4K2-STRIPAKSTRN4 complex, activating MAP4K2. - Autophagy mediated by MAP4K2 is critical for the development of head and neck cancer. |
||
| Related Techniques | ||||
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Endocytosis detection | ECGreen-Endocytosis Detection | |||
| Phagocytosis detection | AcidSensor Labeling Kit – Endocytic Internalization Assay and -Cellstain- Calcein-AM solution | |||
| Mitophagy Detection | Mitophagy Detection Kit and Mtphagy Dye | |||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | |||
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | |||
| Antibody/Protein labeling with fast and high recovery |
Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2 | |||
| Related Applications | ||||
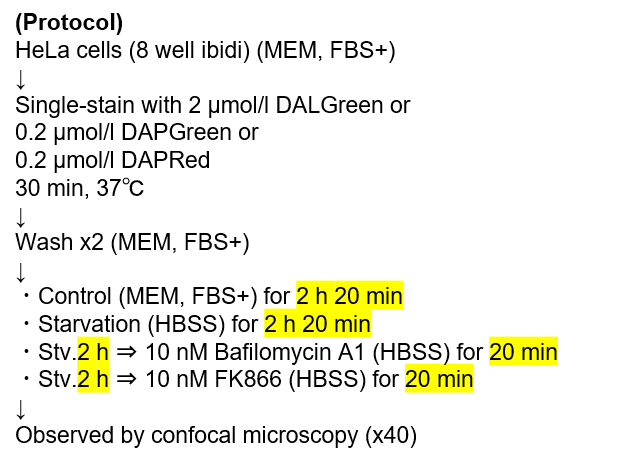
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
<Impact on Lysosomal Acidification and pH Detection>
|
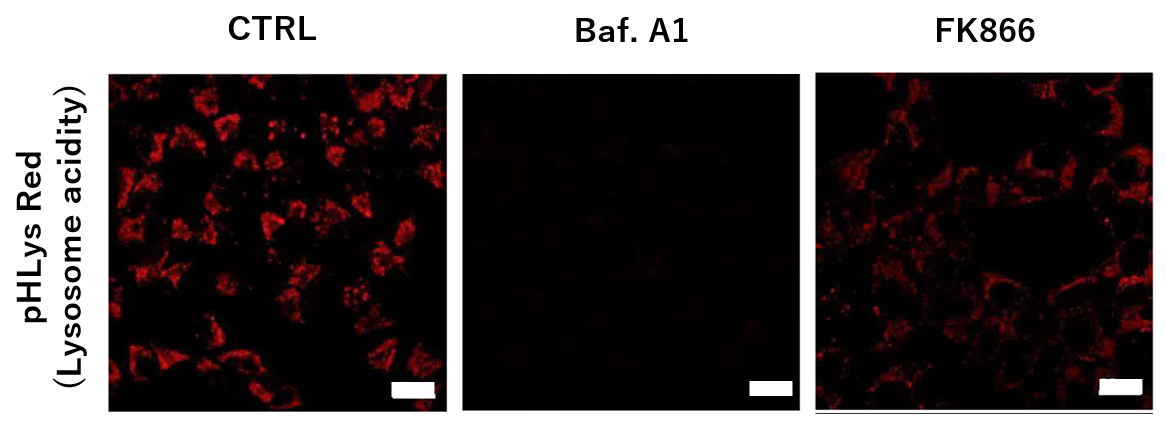
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
|
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
|
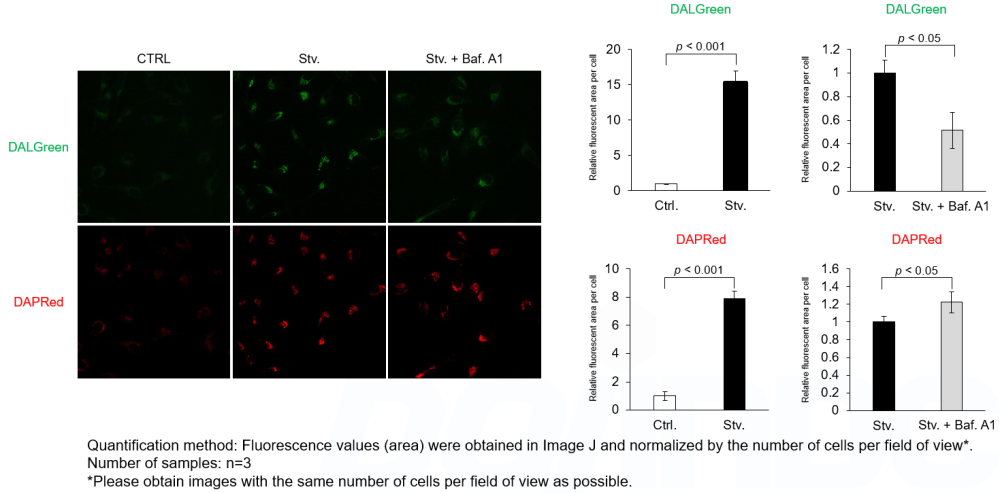
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||
Autophagy for Cellular Homeostasis [Aug. 20, 2024]
Open / Close the Article
|
Cell death and the dysfunction of autophagy and lysosomes are closely linked in maintaining cellular health. When autophagy is impaired, cells cannot effectively degrade and recycle damaged components, leading to the accumulation of toxic materials and cellular stress. Lysosomal dysfunction exacerbates this problem, as these organelles are essential for the degradation of autophagosomal contents. The resulting cellular damage and stress can trigger cell death pathways that contribute to various diseases, including neurodegenerative disorders and cancer. |
||||
| Tumor suppressor Par-4 activates autophagy-dependent ferroptosis Click here for the original article: Karthikeyan Subburayan, et. al., Communications Biology, 2024. |
The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34 Click here for the original article: Rasmus Berglund, et. al., Nature Communications, 2024. |
Autophagy counters inflammation-driven glycolytic impairment in aging hematopoietic stem cells Click here for the original article: Paul Dellorusso, et. al., Cell Stem Cell, 2024. |
||
|
Point of Interest - Upregulation of Par-4 promotes NCOA4-dependent ferritinophagy, a selective autophagy process that degrades ferritin, increasing free iron release, lipid peroxidation, and ROS production. - Par-4 inhibition blocks ferroptosis-driven tumor suppression, highlighting its potential for cancer therapy. |
Point of Interest - This population is found to be dependent on IL-34, a ligand for CSF1R. - Loss of autophagy-dependent microglia leads to neuronal and glial cell death and increased mortality in aged mice exposed to neuroinflammation. - Conversely, IL-34-mediated microglial expansion is protective. |
Point of Interest - Inflammation suppresses glucose uptake and suppresses glycolysis in oHSCs via Socs3 inhibition of AKT/FoxO signaling, but Socs3-mediated autophagy allows metabolic compensation and a shift toward adaptive lipid metabolism. - Fasting/refeeding restores the glycolytic state and enhances the regenerative potential of oHSCs. |
||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit | |||
| Autophagy detection | DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Ferrous ion (Fe2+) detection | FerroOrange(intracellular), Mito-FerroGreen(mitochondrial) | |||
| Glutathione Quantification | GSSG/GSH Quantification Kit | |||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | |||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples. | |||
| Glycolysis/Oxidative phosphorylation Assay | Extracellular OCR Plate Assay Kit, Glycolysis/OXPHOS Assay Kit | |||
| Glucose Uptake Capacity Assay | Glucose Uptake Assay Kit-Blue/ Green/ Red | |||
| Related Applications | ||||
Analysis of autophagic flux without transfection
Experimental Data
Experimental Conditions Procedure Products in Use
|
||||
Induction of autophagy and lysosome dependent cell death [Jun. 18, 2024]
Open / Close the Article
|
Cell death and the dysfunction of autophagy and lysosomes are closely linked in maintaining cellular health. When autophagy is impaired, cells cannot effectively degrade and recycle damaged components, leading to the accumulation of toxic materials and cellular stress. Lysosomal dysfunction exacerbates this problem, as these organelles are essential for the degradation of autophagosomal contents. The resulting cellular damage and stress can trigger cell death pathways that contribute to various diseases, including neurodegenerative disorders and cancer. |
||||
| Copper-dependent autophagic degradation of GPX4 drives ferroptosis Click here for the original article: Qian Xue, et. al., Autophagy, 2023. |
Scd1 and monounsaturated lipids are required for autophagy and survival of adipocytes Click here for the original article: Hiroyuki Mori, et. al., Molecular Metabolism, 2024. |
Glucose starvation causes ferroptosis-mediated lysosomal dysfunction Click here for the original article: Kenji Miki, et. al., iScience, 2024. |
||
|
Point of Interest - On the other hand, exogenous copper directly binds to cysteines of GPX4, leading to ubiquitination and aggregation of GPX4. - Ubiquitinated and aggregated GPX4 induces autophagy via the Tax1 binding protein and is subsequently degraded. - Copper enhances ferroptosis and suppresses tumor progression in a mouse model. |
Point of Interest - Scd1-deficiency leads to lysosomal dysfunction and ultimately to the accumulation of non-acidic autophagosomes. - This results in vacuole accumulation and eventual autophagy-dependent cell death. - Inhibition of autophagosome formation or supplementation with monounsaturated fatty acids maintains the vitality of Scd1-deficient adipocytes. |
Point of Interest - This result leads to dysfunction of lysosomes and accumulation of damaged lysosomes. - Lysosomal dysfunction is mainly caused by several enzymes of the glycolytic and NAD pathways, including ALDOA, GAPDH, NAMPT, and PGK1, which do not function effectively under glucose starvation. - Ferroptosis occurs via iron accumulation due to dysfunction of DMT1, which excretes iron from lysosomes. |
||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit | |||
| Autophagy detection | DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Ferrous ion (Fe2+) detection | FerroOrange, Mito-FerroGreen |
|||
| Lipid droplet detection | Lipid Droplet Assay Kit - Blue / Deep Red | |||
| Mitophagy detection dye | GSSG/GSH Quantification Kit | |||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples. | |||
| Glycolysis/Oxidative phosphorylation Assay | Extracellular OCR Plate Assay Kit, Glycolysis/OXPHOS Assay Kit | |||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | |||
| Related Applications | ||||
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
|
<Impact on Lysosomal Acidification and pH Detection>
|
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.  |
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>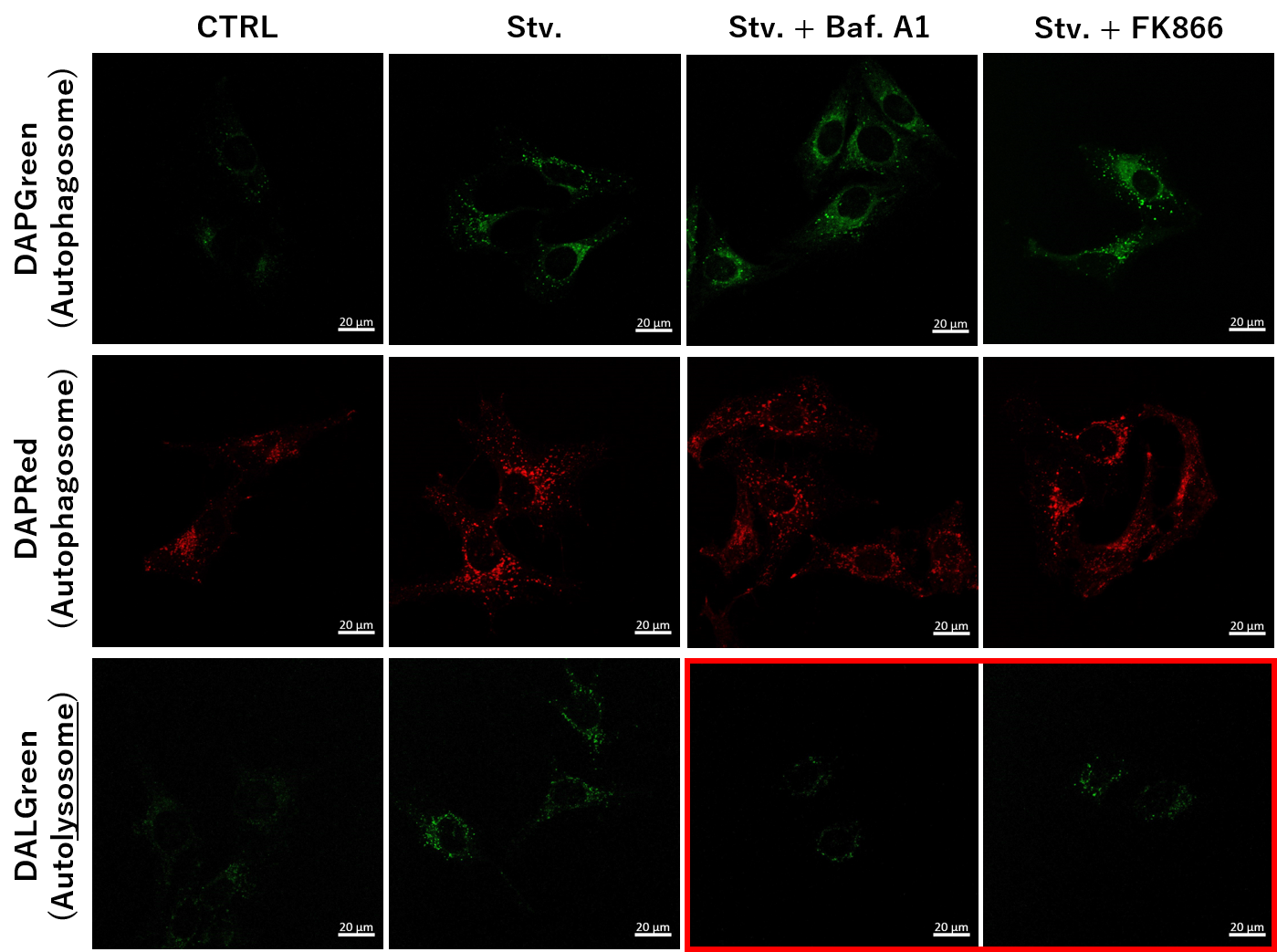 |
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||
Disruption of proteostasis leading to neurodegeneration [May. 14, 2024]
Open / Close the Article
|
Proteostasis refers to the cellular mechanisms that regulate the synthesis, folding, trafficking, and degradation of proteins to maintain overall protein balance and health within the cell. Disruptions in proteostasis, such as misfolded proteins or impaired degradation pathways, can lead to protein aggregates that are toxic to cells, a common feature of neurodegenerative diseases such as Alzheimer's and Parkinson's. Maintaining proteostasis is critical in neurons because their long lifespan and high metabolic activity make them particularly susceptible to the accumulation of protein aggregates. Perturbations in proteostasis can initiate or exacerbate neurodegenerative processes, ultimately contributing to neuronal dysfunction and cell death. |
||||
| Accumulation of APP C-terminal fragments causes endolysosomal dysfunction through the dysregulation of late endosome to lysosome-ER contact sites Click here for the original article: Marine Bretou, et. al., Developmental Cell, 2024. |
NAD depletion mediates cytotoxicity in human neurons with autophagy deficiency Click here for the original article: Congxin Sun, et. al., Cell Reports, 2023. |
Integrative analysis reveals a conserved role for the amyloid precursor protein in proteostasis during aging Click here for the original article: Nature Communications, et. al., Cell Reports, 2023. |
||
|
Point of Interest - APP(amyloid precursor protein) C-terminal fragments (APP-CTFs) localize to late endosome/lysosome-ER contacts and an excess of APP-CTFs reduces lysosomal Ca2+ replenishment from the ER. - Balanced levels of APP-CTF are important for lysosomal homeostasis. |
Point of Interest - Dysfunctional autophagy causes NAD depletion due to hyperactivation of NAD-consuming enzymes. - Increasing intracellular NAD levels rescues the viability of autophagy-deficient neurons. |
Point of Interest - Appl regulated autophagy through TGFβ signaling in mouse genetic, human iPSC and in vivo tauopathy models. - The role of APP in controlling age-dependent proteostasis is conserved and associated with Alzheimer's disease. |
||
| Related Techniques | ||||
| Lysosomal function | Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| First-time autophagy research | Autophagic Flux Assay Kit | |||
| Autophagy detection | DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Mitophagy detection dye | Mitophagy Detection Kit and Mtphagy Dye | |||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples. | |||
| Glycolysis/Oxidative phosphorylation Assay | Extracellular OCR Plate Assay Kit, Glycolysis/OXPHOS Assay Kit | |||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | |||
| Related Applications | ||||
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
|
<Impact on Lysosomal Acidification and pH Detection>
|
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.  |
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response> |
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||
How does cancer exploit the lysosome and autophagy? [Apr. 23, 2024]
Open / Close the Article
|
Autophagy is a cellular process that involves the degradation and recycling of cellular components through the formation of autophagosomes, which subsequently fuse with lysosomes for content degradation. In the context of cancer, autophagy plays a dual role; it can suppress tumor initiation by eliminating damaged organelles and proteins, but can also promote tumor survival and growth under metabolic stress by providing nutrients through the recycling of cellular components. |
||||
|
Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion |
Response to Bruton’s tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy Click here for the original article: James D. Phelan, et. al., Cancer Cell, 2024. |
In vivo CRISPR knockout screen identifies p47 as a suppressor of HER2+ breast cancer metastasis by regulating NEMO trafficking and autophagy flux Click here for the original article: Mingang Hao, et. al., Cell Reports, 2024. |
||
|
Point of Interest - Carnosine controls lysosomal subcellular distribution, acidification, and activity by maintaining intracellular pH homeostasis. - By maintaining lysosomal activity, carnosine facilitates NFX1 degradation, which triggers galectin-9 and T-cell-mediated immune escape and tumorigenesis. |
Point of Interest -Chronic selective autophagy suppresses NF-κB signaling in MCD-type Diffuse large B cell lymphoma (DLBCL). -MCD-type DLBCL develops genetic and epigenetic alterations that attenuate selective autophagy. -Disruption of selective autophagy leads to the accumulation of ubiquitinated MYD88L265P. -BTK and mTOR inhibitors interfere with the My-T-BCR pathway by enhancing the autophagic degradation of MYD88L265P. |
Point of Interest -An in vivo CRISPR screen identifies p47 as a suppressor of HER2+ breast cancer metastasis. -Depletion of p47 reduces NEMO endosomal trafficking and enhances NF-κB signaling. -Ablation of p47 impairs lysosomal repair and autophagic flux, leading to increased metastasis. -Lower expression of p47 correlates with increased metastasis in human breast cancer. |
||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit |
|||
| Autophagy detection dyes for imaging | DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Autophagy detection for Flow cytometry / plate assay | DAPGreen (Autophagosome detection) | |||
| Mitophagy detection dye | Mitophagy Detection Kit and Mtphagy Dye | |||
| Lysosomal pH and mass detection | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red |
|||
| Accurate detection of endocytosis by pH changes | ECGreen-Endocytosis Detection | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |||
| Related Applications | ||||
Analysis of autophagic flux without transfection |
||||
|
Detection Principle DALGreen and DAPRed labeled HeLa cells were used to evaluate changes in autophagic flux induced by the lysosomal acidification inhibitor bafilomycin A1 (Baf. A1). Compared to starvation conditions, the fluorescence signals of DALGreen were decreased under inhibited conditions of autolysosome formation by the addition of Baf. A1. In contrast, the fluorescence signals of DAPRed were increased under the same conditions, indicating that Baf. A1 led to the accumulation of autophagosome. Experimental Data
Experimental Conditions Procedure Products in Use |
||||
Autophagic Responces in Neurodegeneration and Aging [Apr. 16, 2024]
Open / Close the Article
|
Autophagy plays a critical role in neurodegeneration and aging by maintaining cellular health through the degradation and recycling of damaged cellular components. In the context of neurodegeneration, such as Alzheimer's, Parkinson's and Huntington's diseases, impaired autophagy contributes to the accumulation of toxic protein aggregates that exacerbate neuronal damage and dysfunction. Conversely, enhancing autophagy has been shown to mitigate these deleterious effects and improve neuronal survival, suggesting a protective role in aging and neurodegenerative diseases. Research continues to explore autophagy modulators as potential therapeutic agents to slow the progression of neurodegenerative diseases and promote healthier aging. |
||||
|
The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34 |
Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging Click here for the original article: Juan Ignacio Jiménez-Loygorri, et. al., Nature Communications, 2024. |
SKA2 regulated hyperactive secretory autophagy drives neuroinflammation-induced neurodegeneration Click here for the original article: Jakob Hartmann, et. al., Nature Communications, 2024. |
||
|
Point of Interest - This population is found to be dependent on IL-34, a ligand for CSF1R. - Loss of autophagy-dependent microglia leads to neuronal and glial cell death and increased mortality in aged mice exposed to neuroinflammation. - Conversely, IL-34-mediated microglial expansion is protective. |
Point of Interest - There is a marked upregulation of the type I interferon response in the retinas of older mice. - This response correlates with increased levels of cytosolic mtDNA and activation of the cGAS/STING pathway. - In older mice, induction of mitophagy with urolithin A attenuates cGAS/STING activation and ameliorates the deterioration of neurological function. |
Point of Interest - Hippocampal Ska2 knockdown in male mice hyperactivates secretory autophagy (SA), leading to neuroinflammation and subsequent neurodegeneration. - Hyperactivation of SA increases IL-1β release, contributing to an inflammatory feed-forward vicious cycle including NLRP3 inflammasome activation and Gasdermin D-mediated neurotoxicity. - Results from protein analyses of postmortem human brains show that SA is hyperactivated in Alzheimer's disease. |
||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit |
|||
| Autophagy detection dyes for imaging | DAPRed (Autophagosome detection) DALGreen (Autolysosome detection) |
|||
| Autophagy detection for Flow cytometry / plate assay | DAPGreen (Autophagosome detection) | |||
| Mitophagy detection dye | Mitophagy Detection Kit and Mtphagy Dye | |||
| Lysosomal pH and mass detection | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red |
|||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples. | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |||
| Related Applications | ||||
Analysis of Lysosomal Mass and pH Exchange in Senescence-induced Cells |
||||
|
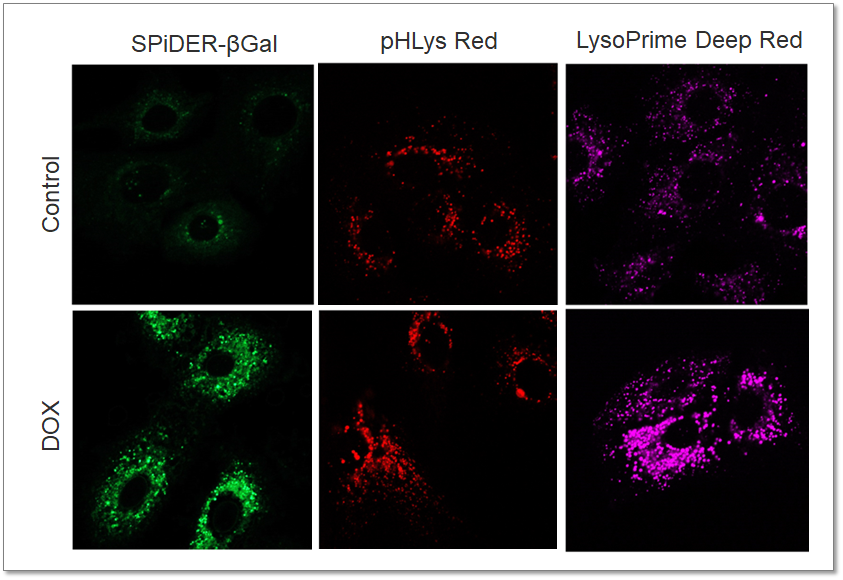
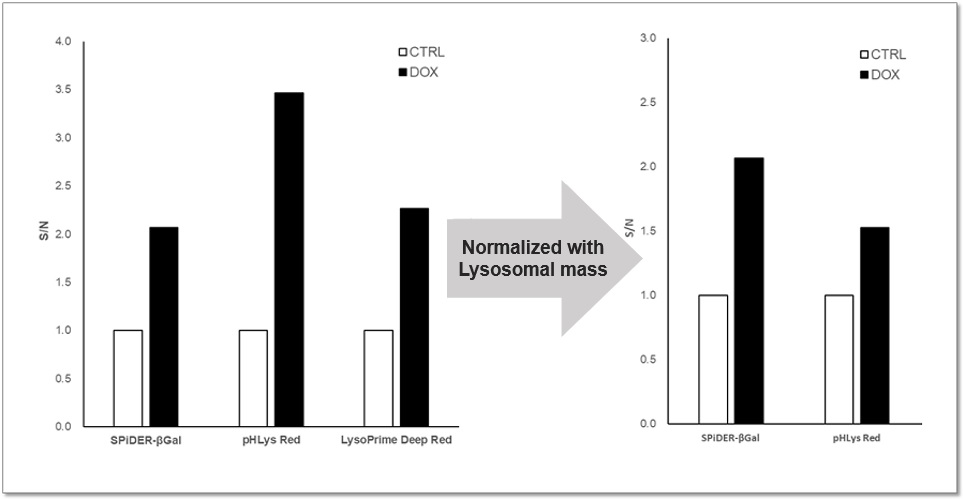
Purpose: To investigate changes in lysosomal mass and pH in A549 cells induced to senescence by treatment with Doxorubicin (DOX). Methods: Senescence-associated acidic β-galactosidase (SA-βGal) activity was detected using Cellular Senescence Detection Kit - SPiDER-βGal. Lysosomal mass was detected using LysoPrime Deep Red, and pH was detected using pHLys Red. Fluorescence imaging was used to observe changes in lysosomal mass and pH in senescent cells compared to non-senescent cells. The normalized fluorescence intensity of lysosomal mass and pH was also measured by a plate reader. Results: Our findings indicate that senescence induced by DOX resulted in an increase in lysosomal mass and acidification of pH compared to non-senescent cells. The obtained results are consistent with previous reports* that demonstrated enhanced lysosomal activity in senescent cells induced by the CDK4/6 inhibitor, palbociclib. The fluorescence imaging and plate reader data both support these findings. * Miguel Rovira, et. al., Aging Cell (2022) <Experimental Conditions for Microscopy> <Experimental Conditions for Plate Reader> <Products in Use>
|
|
|||
Monounsaturated lipids Regulate Adipocyte Viability via Autophagy [Apr. 9, 2024]
Open / Close the Article
|
Scientists have revealed that Scd1, which is induced by exposure to cool temperatures, and monounsaturated lipids are crucial for the survival of adipocytes. Inhibition of Scd1 leads to autophagy and cell death, while supplementation with monounsaturated fatty acids can prevent this. |
||||
|
Scd1 and monounsaturated lipids are required for autophagy and survival of adipocytes |
||||
|
Point of Interest - Blockade of autophagy or supplementation with monounsaturated fatty acids rescues Scd1-deficient adipocytes from death. - Reduced bone marrow adipocyte volume and number in mice deficient for Scd1 specifically in bone marrow adipocytes. - Scd1 deficiency causes cell death characterized by the accumulation of autophagosomes due to lysosomal dysfunction. |
||||
| Related Techniques | ||||
| First-time autophagy research | Autophagic Flux Assay Kit |
|||
| Autophagy detection dyes for imaging | DAPRed (Autophagosome detection) DALGreen (Autolysosome detection) |
|||
| Autophagy detection dyes for Flow cytometry and plate reader | DAPGreen (Autophagosome detection) | |||
| Mitophagy detection dye | Mitophagy Detection Kit and Mtphagy Dye | |||
| Lysosomal pH and mass detection | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red |
|||
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |||
| Related Applications | ||||
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
<Impact on Lysosomal Acidification and pH Detection>
|
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
|
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
|
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||
Microglial and Neuronal Autophagy [Mar. 19, 2024]
Open / Close the Article
|
Autophagy is a cellular process that degrades and recycles damaged organelles, misfolded proteins, and other cellular debris, and is critical for maintaining cellular homeostasis. Dysregulated autophagy is often observed in the context of neurodegeneration, where either excessive or insufficient autophagic activity can contribute to the accumulation of toxic proteins and organelle dysfunction that are hallmarks of neurodegenerative diseases. Enhancing autophagy has been shown to alleviate symptoms and pathology in models of diseases such as Alzheimer's, Parkinson's, and Huntington's, suggesting a protective role against neurodegeneration. Conversely, excessive autophagy can lead to cell death, suggesting that a delicate balance is required for neuronal health and highlighting the complexity of targeting autophagy as a therapeutic strategy for neurodegenerative diseases. |
|||||
| Autophagy enables microglia to engage amyloid plaques and prevents microglial senescence Click here for the original article: Insup Choi, et. al., Nature Cell Biology, 2023. |
Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration Click here for the original article: Beatrice Paola Festa, et. al., Neuron, 2023. |
Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease Click here for the original article: Lauren H. Fairley, et. al., PNAS, 2022. |
|||
|
Point of Interest - An autophagy deficiency fosters the development of senescent microglia. - Pharmacological removal of autophagy-deficient senescent microglia can alleviate neuropathology in mice with AD. |
Point of Interest - CCL-5, CCL-4, and CCL-3 derived from microglia activate the neuronal CCR5 receptor, leading to the inhibition of neuronal autophagy. - Levels of CCR5, CCL-3, CCL-4, and CCL-5 are elevated in the brains of mice with Huntington’s disease and tauopathy. - Inhibiting CCR5, either pharmacologically or genetically, ameliorates neurodegeneration in mice. |
Point of Interest - Hexokinase-2 (HK) affects glycolytic metabolism and phagocytosis through its interaction with mitochondria. - Mitochondrial HK influences glycolysis and inflammation, and its displacement improves phagocytosis in TSPO-deficient microglia. - Alzheimer’s beta-amyloid drastically stimulated mitochondrial HK recruitment in cultured microglia, which may contribute to microglial dysfunction in Alzheimer’s disease. |
|||
| Reported Examples: Autophagy and Mitophagy Detection Probes | |||||
| Detection (Reagent) | Samples | Reference | |||
| Autophagosome/Autolysosome (Autophagic Flux Assay Kit) |
Immortalized Fischer rat Schwann cells | International Journal of Molecular Sciences | |||
|
Autolysosome |
Cortical neurons | Neurobiology of Disease | |||
| PC12 cells(a mixture of neuroblastic cells and eosinophilic cells) | Hindawi | ||||
| Autophagosome (DAPGreen or DAPRed) |
Neurons from mouse embryonic stem cells | Life Science Alliance | |||
| Hippocampal neurons | PNAS | ||||
| N2a cells (Mouse Albino neuroblastoma) | International Journal of Molecular Sciences | ||||
| Mitophagy (Mtphagy Dye) |
Neural progenitor cell (NPC) | International Journal of Molecular Sciences | |||
| Primary cortical neuronal (CxN) cells | Cells | ||||
| Brain slices (mice) | The FEBS Journal | ||||
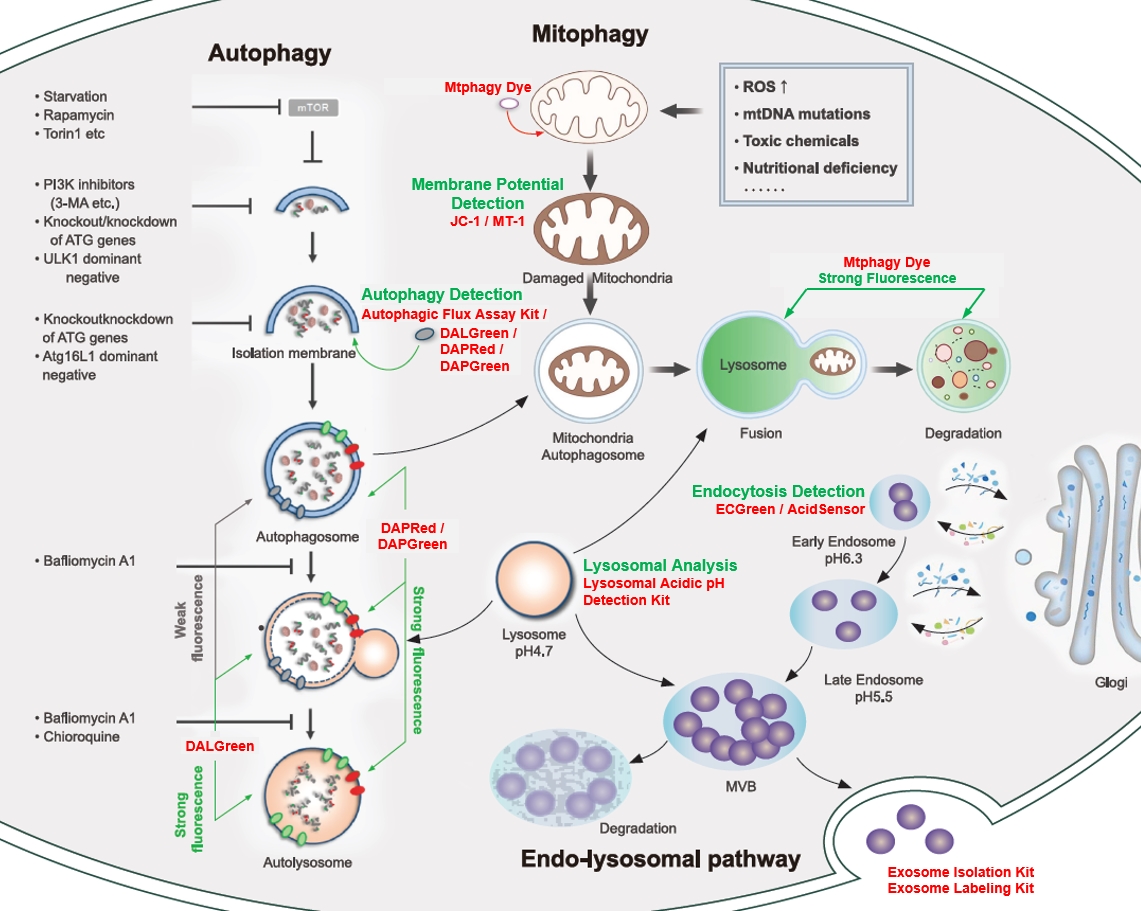
<Cellular Landscape: autophagy-related pathway>
|
Mitophagy Detection Mitochondrial Membrane Potential Detection Lysosomal Analysis Endocytosis Detection Exosome |
||||
New Insights into Autophagic and Endocytic Pathways [Feb. 21, 2023]
Open / Close the Article
| Autophagy is a cellular process that involves the degradation and recycling of cellular components such as damaged organelles, misfolded proteins, and intracellular pathogens. Autophagy is regulated by a complex network of several pathways, including the endocytic pathway. The endocytic pathway is responsible for the trafficking and sorting of proteins and lipids between different cellular compartments, including the plasma membrane, endosomes, lysosomes, and the trans-Golgi network. These pathways sometimes cooperate and sometimes independently contribute to cellular homeostasis. Today, we introduce you to three highlighted articles related to Autophagic and Endocytic pathways focusing on Nucleophay and aging, Endosomal lipid signaling, and Autophagy and the circadian clock. | ||
|---|---|---|
| Nucleophagy and aging | Endosomal lipid signaling | Autophagy and the circadian clock |
| Nucleophagy delays aging and preserves germline immortality (Margarita-Elena Papandreou, et al., Nature Aging, 3, 34-46, 2022) |
Endosomal lipid signaling reshapes the endoplasmic reticulum to control mitochondrial function (Wonyul Jang, et al., Science, 378, 6625, 2022) |
Reciprocal regulation of chaperone-mediated autophagy and the circadian clock (Yves R. juste, et al., Nature Cell Biology, 23, 1255-1270, 2021) |
|
|
|
| Related Technique in This Topic | ||
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |
| Endocytosis detection | ECGreen | |
| Endocytic internalization assay | AcidSensor Labeling Kit | |
| Lysosomal function assay | Lysosomal pH and mass detection Kit | |
| Extracellular vesicles labeling | ExoSparkler Exosome Membrane Labeling Kit-Green, Red, Deep Red | |
| Extracellular vesicles Isolation | ExoIsolator Exosome Isolation Kit | |
| Cellular senescence detection (Live cell imaging or FCM) |
Cellular Senescence Detection Kit - SPiDER-βGal | |
| Cellular senescence detection (Plate reader) | Cellular Senescence Plate Assay Kit - SPiDER-βGal | |
Autophagic Stress Responses [Jan. 23, 2024]
Open / Close the Article
|
Autophagy is a cellular process that degrades and recycles damaged cellular components and is critical for maintaining cellular health and function. In neurons, autophagy plays an important protective role by removing defective proteins and organelles, preventing the accumulation of toxic aggregates that can lead to neurodegenerative diseases. This process also helps maintain synaptic plasticity and neuronal homeostasis, which are essential for proper neuronal function and brain health. In addition, by regulating the response to neural stress, autophagy contributes to the overall resilience and longevity of neural cells. |
||||
| The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34 Click here for the original article: Rasmus Berglund, et. al., Nature Communications, 2024. |
Lysophagy protects against propagation of α-synuclein aggregation through ruptured lysosomal vesicles Click here for the original article: Keita Kakuda, et. al., PNAS, 2023. |
The Hippo pathway noncanonically drives autophagy and cell survival in response to energy stress Click here for the original article: Gayoung Seo, et. al., Molecular Cell, 2023. |
||
|
Point of Interest - This population is found to be dependent on IL-34, a ligand for CSF1R. - Loss of autophagy-dependent microglia leads to neuronal and glial cell death and increased mortality in aged mice exposed to neuroinflammation. - Conversely, IL-34-mediated microglial expansion is protective. |
Point of Interest - This rupture seeded the aggregation of endogenous αSyn, initially around damaged lysosomes. - Exogenous αSyn aggregates induced the accumulation of LC3 on lysosomes, indicating the activation of lysophagy. - Autophagy-deficient cells treated with αSyn aggregates had an increased number of ruptured lysosomes and enhanced propagation of αSyn aggregates. |
Point of Interest - MAP4K2 phosphorylates LC3A at Serine 87 to promote autophagic flux. - Energy stress induces the formation of the MAP4K2-STRIPAKSTRN4 complex, activating MAP4K2. - Autophagy mediated by MAP4K2 is critical for the development of head and neck cancer. |
||
| Related Techniques | ||||
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Lysosomal function | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Endocytosis detection | ECGreen-Endocytosis Detection | |||
| Phagocytosis detection | AcidSensor Labeling Kit – Endocytic Internalization Assay and -Cellstain- Calcein-AM solution | |||
| Mitophagy Detection | Mitophagy Detection Kit and Mtphagy Dye | |||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | |||
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | |||
| Antibody/Protein labeling with fast and high recovery |
Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2 | |||
| Related Applications | ||||
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
<Impact on Lysosomal Acidification and pH Detection>
|
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
|
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
|
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||
Autophagy converts developing neural stem cells to the adult quiescent state [Dec. 19, 2023]
Open / Close the Article
|
Scientists have discovered that protein aggregates and components of the autophagy machinery accumulate in developmental radial glia-like neural stem cells (NSCs) as they enter quiescence. They also uncover an important role of autophagy in the transition of developmental NSCs to their quiescent adult form by modulating autophagy activity in vivo. |
||||
|
Autophagy drives the conversion of developmental neural stem cells to the adult quiescent state |
||||
|
Point of Interest |
||||
| Related Techniques | ||||
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) | |||
| Cell cycle assay | Cell Cycle Assay Solution Deep Red and Blue | |||
| Lysosomal pH and mass detection | Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | |||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | |||
| Related Applications | ||||
Tracing autophagosome to autolysosome in live cellsNampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021) |
||||
<Impact on Lysosomal Acidification and pH Detection>
|
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
|
|||
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
|
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
|
|||