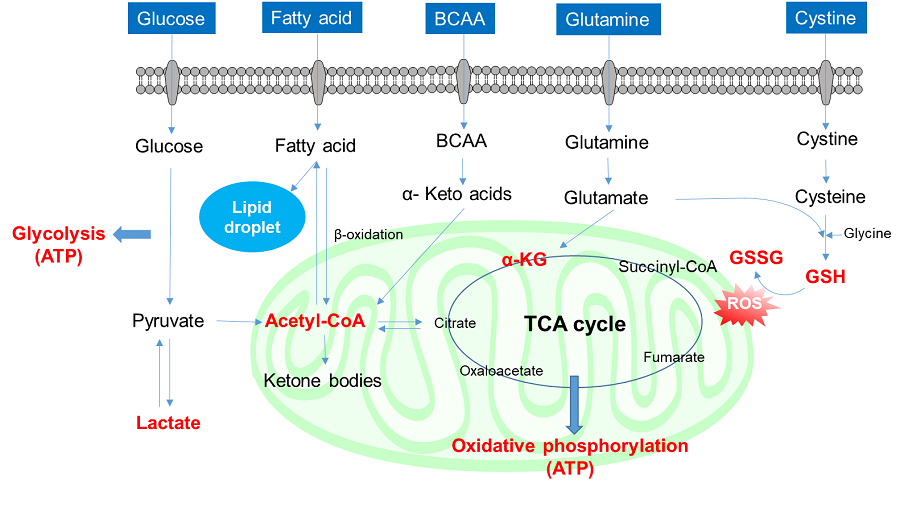
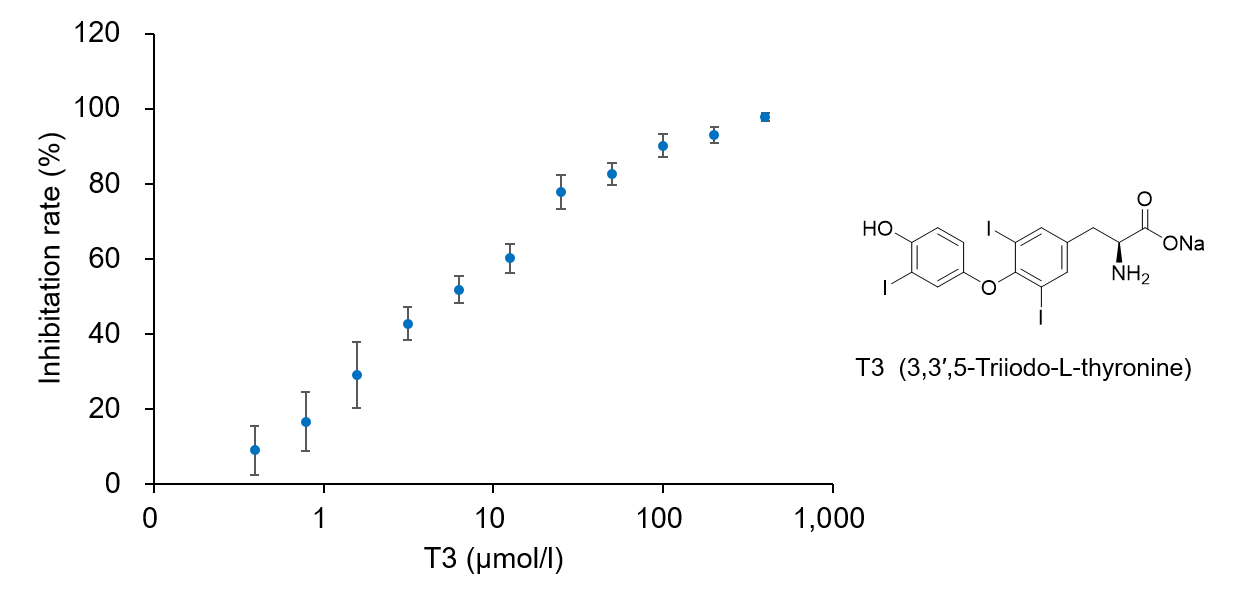
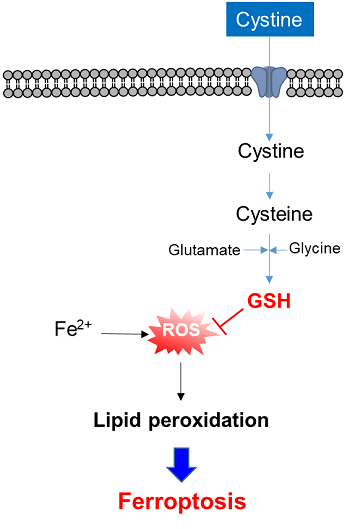
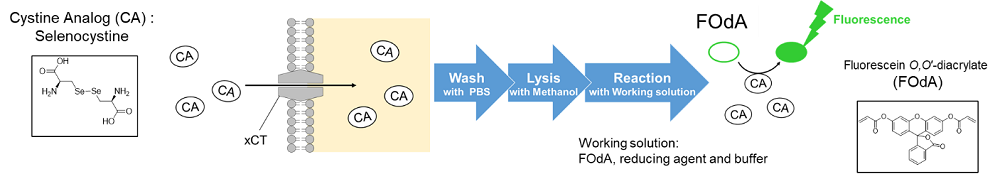
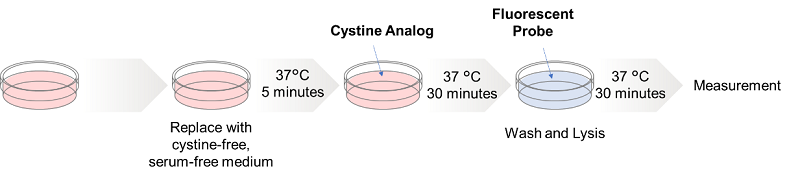
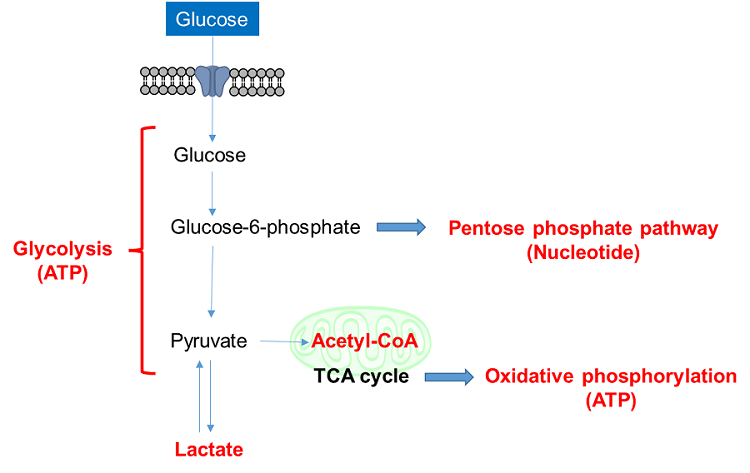
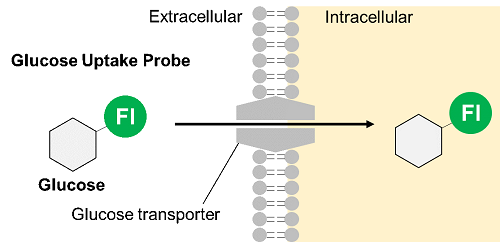
Glucose is one of the most important nutrients and is taken up into cells mainly via glucose transporters (GLUTs ). Intracellular glucose is a major source of ATP, nucleic acids, and lipids. After glucose is taken into the cell, it is metabolized by various enzymes and is converted to pyruvate via the glycolytic pathway. Then pyruvate participates in the citric acid circuit (TCA cycle ) via acetyl-CoA in the mitochondria and is used to produce NADH and FADH, which are necessary for ATP production. Although ATP can be synthesized by both the glycolysis pathway and the OXPHOS pathway, OXPHOS using NADH and FADH in mitochondria is the most efficient and major ATP production pathway.
Cancer cells uptake large amounts of glucose to maintain their high proliferative capacity. Because cancer cells mainly use the glycolytic pathway to produce ATP, it has been a classic anti-cancer drug target. Although effective anticancer drugs have not yet been developed, the glycolytic pathway is still a major drug target in cancer cells and is the most important pathway for understanding cancer cell metabolism.
1-1. Glucose uptake assay
Radiolabeled glucose is the most common method of evaluating the glucose uptake capacity of cells. However, because of the radioactive compound, it is complicated to perform and lacks versatility. An enzymatic cycling method using 2-deoxy-D-glucose (2-DG ) can be used for colorimetric or fluorescent plate assays, but it is not applicable for cell imaging or flow cytometry. The fluorescent glucose tracer 2-NBDG has recently been more widely used, but its low fluorescence intensity is widely criticized.
Thus, Dojindo Laboratories have developed a new fluorescent-labeled glucose derivative, Glucose Uptake Probe (Blue, Green, Red), to replace 2-NBDG. The Glucose Uptake Probe is much brighter than 2-NBDG and can measure the glucose uptake capacity of cells with high sensitivity. In addition, there are three different colors to meet different needs, enabling its use in various applications.

| Category |
Product Name(Click the links in the table to view the product details) |
| Glucose Uptake |
Glucose Uptake Assay Kit-Blue, Green, Red |
1-2. Application: Glucose uptake of cancer cells
Inhibition of glucose uptake by glucose transporter inhibitors
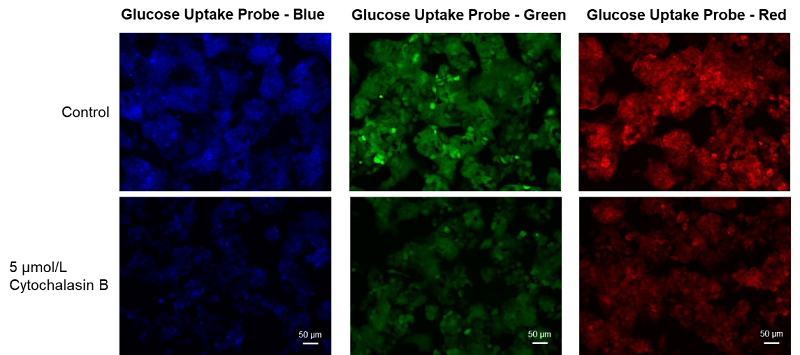
Cell line: Human liver cancer cell, HepG2
Stimulation: Glucose transporter inhibitor, cytochalasin B
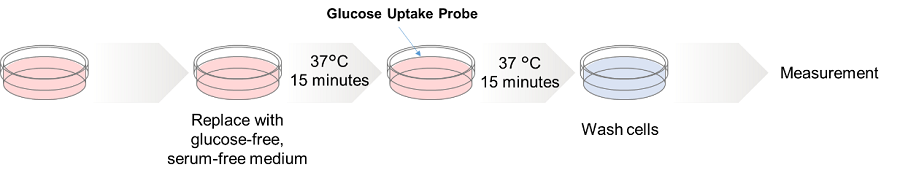
HepG cells (human hepatocellular carcinoma cells) were treated with cytochalasin B and the changes in the glucose uptake capacity of the cells were observed by fluorescence microscopy using the Glucose Uptake Probe. This approach confirmed that the glucose uptake ability of the cells was inhibited by cytochalasin B treatment.
Glucose uptake inhibition by reagents
Provided by Prof. Hiroshi Maeda (Founder of BioDynamics Laboratory Inc.)
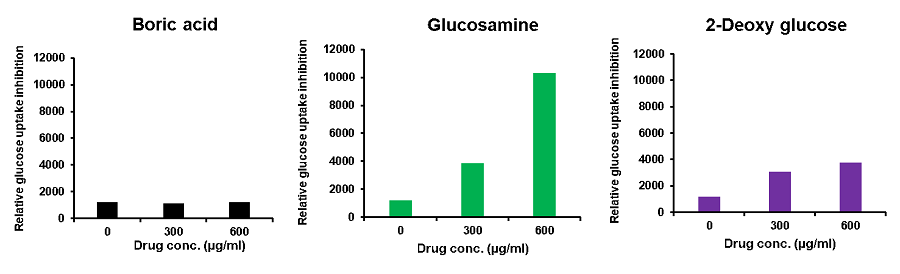
Cell line: Mouse colon cancer cells, Colon26
Reagents: Boric acid, glucosamine, 2-deoxy-D-glucose
Colon26 cells (mouse colon cancer cells) were treated with various reagents, and the glucose uptake of the cells was measured using the Glucose Uptake Probe-Green. The results demonstrated that glucose uptake was inhibited by the addition of the reagents, and the effect varied depending on these reagents.
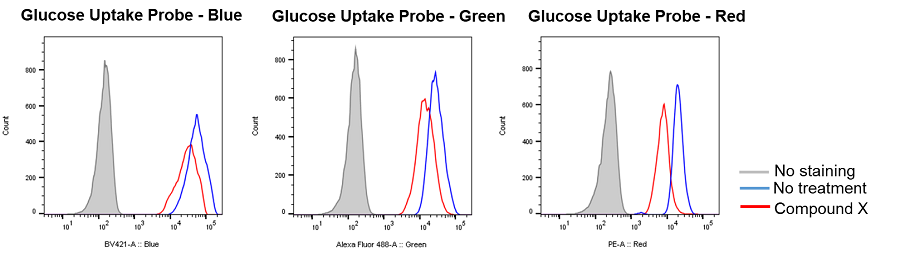
Inhibition of glucose uptake by compounds that inhibit the glycolytic pathway
Provided by Prof. Heiichiro Udono (Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences)
Cell line: Mouse lung cancer cells, 3LL
Reagents: Glycolytic pathway inhibitor (not disclosed, referred to as Compound X)
3LL cells were treated with Compound X, which inhibits the glycolytic pathway, and the glucose uptake of the cells was measured by flow cytometry using the Glucose Uptake Probe. The results demonstrated that the glucose uptake of the cells was inhibited by Compound X.
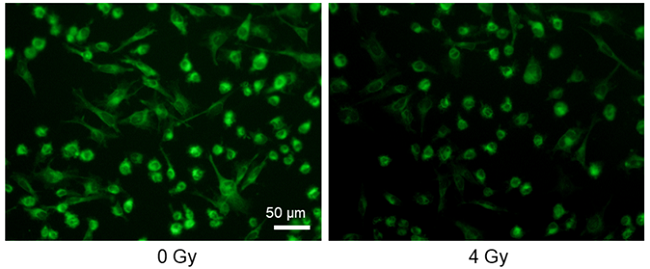
Decreased glucose uptake by X-ray irradiation
Provided by Assistant Prof. Shinya Kato (Mie University Advanced Science Research Promotion)
Cell line: Astrocytoma cell line, U-251MG
Stimulation: X-ray irradiation
X-ray irradiation of U-251MG cells decreased the glucose uptake capacity of the cells.
1-3. Application: Glucose uptake by immune cells
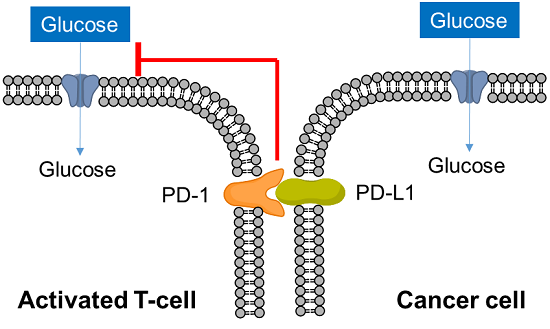
T cells play a central role in the immune system to eliminate cancer cells. In recent years, it has become clear that metabolism is also involved in the regulation of T cell functions such as differentiation and activation, and research on metabolism in cancer immunity has become more popular. Cancer cells absorb large amounts of nutrients to maintain their proliferative activity, while activated T cells also require large amounts of nutrition, especially glucose, to eliminate cancer cells. Therefore, activated T cells and cancer cells compete for glucose uptake.
Cancer cells express PD-L1 to suppress T cell activity in response to PD-1 on the surface of activated T cells, an immune checkpoint molecule, and recent studies have shown that this interaction suppresses T cell glucose uptake. Cancer cells also regulate the metabolism of immune cells to evade the immune system in this way, and it is important to understand the metabolism of immune cells as well as cancer cells in the field of cancer immunology.
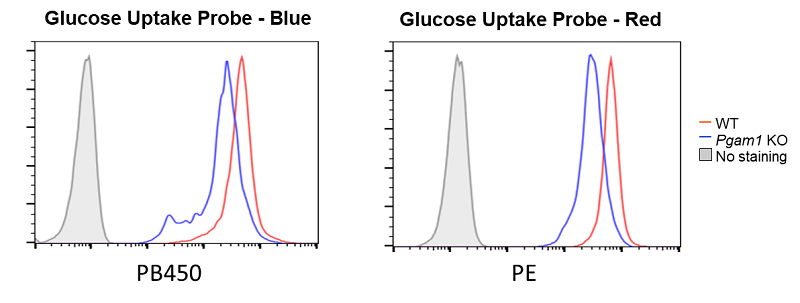
Decreased glucose uptake due to deficiency of the glycolytic enzyme Pgam1
Provided by Prof. Masakatsu Yamashita (Ehime University Department of Immunology, Graduate School of Medicine)
Cell line: Mouse activated T cells, CD4+ T cell, with deficiency of the glycolytic enzyme Pgam1
Decreased glucose uptake was observed in Pgam1-deficient CD4+ T cells (mouse activated T cells).
1-4. Application: Glucose uptake in adipocytes
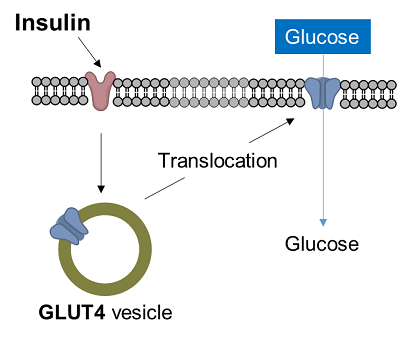
Diabetes is a disease in which glucose in the blood is not taken up into the cells, resulting in increased blood glucose levels. In muscle cells and adipocytes, insulin stimulation causes GLUT4, a glucose transporter, to migrate to the surface of the cell membrane and promote glucose uptake. This insulin-responsive glucose uptake is closely related to diabetes and is an important target in the clarification of diabetes and the development of therapies.
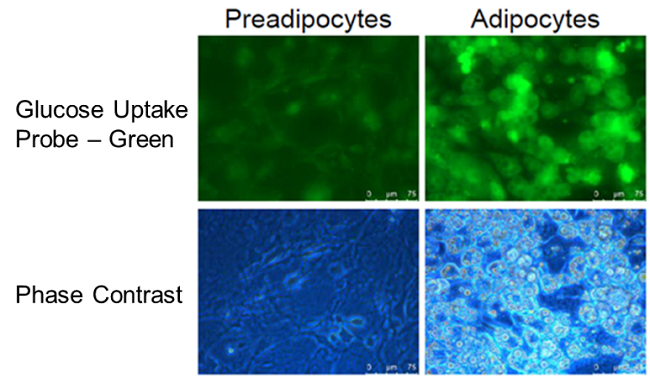
Enhancement of glucose uptake during differentiation into adipocytes
Provided by Associate Prof. Yoshihiro Matsumura (Tokyo University, Research Center for Advanced Science and Technology)
Cell line: Mouse progenitor adipocytes, 3T3L1
The glucose uptake capacity of mouse progenitor adipocytes increased as long as differentiation into adipocytes was observed.
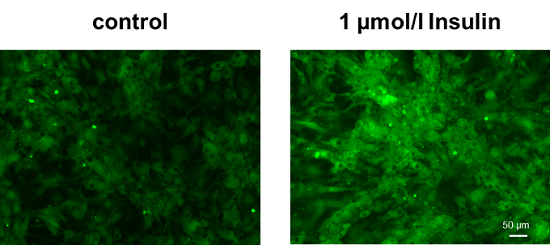
Enhancement of glucose uptake in adipocytes after insulin treatment
Cell line: Mouse progenitor adipocytes, 3T3L1
Insulin treatment of adipocytes differentiated from mouse progenitor adipocytes enhanced glucose uptake.