General Information
Endocytosis is a cellular process that involves macromolecules being taken up through a plasma membrane-derived vesicle called an endosome. This process contributes to the immune response, cell signaling, elimination of apoptotic cells, and intracellular homeostasis such as bringing various nutrients to the cell and transporting unwanted components to the lysosome1). AcidSensor Labeling Kit – Endocytic Internalization Assay is used for labeling pH-sensitive fluorescent probe. NH2-Reactive AcidSensor, a component of this kit, has a succinimidyl ester group, and can easily make a covalent bond with an amino group of the target molecules (e.g., proteins and antibodies) without any activation process. The fluorescence intensity of the AcidSensor is enhanced as the acidity increases, whereas it is weak in neutral conditions. Therefore, AcidSensor-labeled molecules internalized via endocytosis shows fluorescence in acidic condition like lysosome. Additionally, AcidSensor emits 633 nm, allowing multiple imaging using green fluorescence, red fluorescence etc.
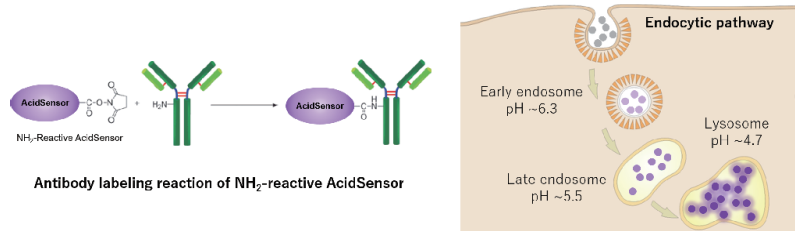
Figure 1. Endocytic pathway and labeling principle of AcidSensor.
Contents
| NH2-Reactive AcidSensor | 3 tubes |
| WS Buffer | 4 ml x 1 |
| Reaction Buffer | 500 µl x 1 |
| Filtration Tube | 3 tubes |
Storage Conditions
Store in a cool (0–5°C), dark, and dry place.
- After an NH2-Reactive AcidSensor is taken out from the sealed bag, keep the unused NH2-Reactive AcidSensor (s) in the bag, seal tightly and store at -20°C. Store the other components at 0-5°C.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Growth medium (serum containing medium or serum-free medium)
- Hanks’ Balanced Salt Solution (HBSS) or basal medium (medium without serum)
- Centrifuge
- Micropipette
- Microtube
Precaution
- Sample with an amino group and molecular weight larger than 50,000 is recommended.
- Use a purifi ed target protein for AcidSensor labeling because other proteins with a molecular weight larger than 10,000, such as serum albumin or gelatin might interfere with the labeling reaction.
- If the protein solution contains small insoluble materials, centrifuge the solution, and use the supernatant for the labeling.
- The droplets induced from the dry inhibitor of the membrane are occasionally found inside Filtration Tube while storing the kit at 0-5℃ or after returning to room temperature. This phenomenon does not aff ect performance.
General Protocol
Preparation of AcidSensor-labeled protein
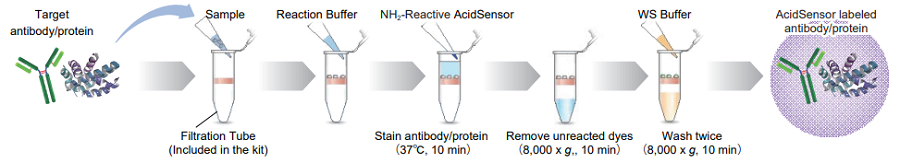
Figure 2. Protocol of AcidSensor labeling.
- Add 100 µl WS Buff er and the sample solution containing 50-200 µg protein to a Filtration Tube.
- The volume of the protein solution should be less than 100 µl. If the protein concentration is lower than 0.5 mg/ml, repeat Steps 1 and 2 until the total protein accumulation becomes 50 - 200 µg.
- Mix the solution with pipetting several times, and centrifuge at 8,000 x g for 10 min.
- If the solution still remains on the membrane after the centrifugation, spin for another 5 min.
- Discard the filtrate.
- Add 10 μl of DMSO to the provided tube containing NH2-Reactive AcidSensor and dissolve by vortex mixer.
- NH2-Reactive AcidSensor can be hydrolyzed by moisture in DMSO. Proceed to Step 5 immediately after the preparation of the NH2-Reactive AcidSensor solution.
- Add 100 µl Reaction Buffer to the Filtration Tube, and then add the total volume of NH2-Reactive AcidSensor DMSO solution (step 4) to the Filtration Tube and pipette to mix.
- Incubate the tube at 37℃ for 10 min.
- Add 100 µl WS Buffer to the Filtration Tube, and centrifuge at 8,000 x g for 10 min.
- If the solution still remains on the membrane after the centrifugation, spin for another 5 min.
- Discard the filtrate.
- Add 200 µl WS Buffer to the Filtration Tube, and centrifuge at 8,000 x g for 10 min. Repeat this step one more time.
- If the solution still remains on the membrane after the centrifugation, spin for another 5 min.
- Add 100 µl WS Buffer, and pipette about 10 times to recover the conjugate. Transfer the solution to a microtube (not included in this kit).
- We recommend using WS Buffer to recover the conjugate. You can choose any kind of buffers appropriate for your experiment.
Addition of AcidSensor-labeled protein to cultured cell
- Seed cells in a dish and culture them overnight at 37℃ in an incubator equilibrated with 95% air and 5% CO2.
- Discard the supernatant and wash the cells with a 200 μl basal medium.
- As necessary, HBSS or growth medium can be used instead of basal medium.
- Discard the supernatant and add 200 µl basal medium. Note: As necessary, HBSS or growth medium can be used instead of basal medium.
- Add 10 μl AcidSensor-labeled protein to the dish containing the cells.
- Optimal concentration of AcidSensor-labeled protein varies depending on the cell type.
- Incubate them for 1-24 hours at 37℃ in an incubator equilibrated with 95% air and 5% CO2.
- Protect from light during the incubation.
- Discard the supernatant and wash the cells twice with 200 µl basal medium.
- As necessary, HBSS or growth medium can be used instead of basal medium.
- Add growth medium to the dish, then observe the cells under a fluorescence microscope.
Usage Example
Preparation of AcidSensor-labeled BSA and Mouse IgG
- BSA or Mouse IgG (1 mg/ml, 100 µl ), and 100 µl WS buffer was added to a Filtration Tube.
- The solution was mixed several times with pipetting , and the Filtration Tube was centrifuged at 8,000 x g for 10 min. The filterate was discarded.
- After 10 µl DMSO was added in a tube containing NH2-Reactive AcidSensor, the solution was mixed by vortex mixer.
- The total volume of NH2-Reactive AcidSensor DMSO solution (step 3) and 100 µl Reaction Buffer were added to the Filtration Tube and mixed with pipetting.
- The Filtration Tube was incubated at 37℃ for 10 min.
- WS Buffer (100 µl) was added to the Filtration Tube, and the Filtration Tube was centrifuged at 8,000 x g for 10 min. The filterate was discarded.
- WS Buffer (200 µl) was addded to the Filtration Tube, and the Filtration tube was centrifuged at 8,000 x g for 10 min. This step was repeated one more time.
- WS Buffer (200 µl) was added and mixed about 10 times with pipetting to recover the conjugate. The solution was transfered to a microtube.
Internalization of the AcidSensor-labeled proteins (BSA or Mouse IgG) in HeLa cells
- HeLa cells (1.0 × 104 cells/well) in minimum essential media (MEM) containing 10% FBS were seeded on a µ-slide 8 well plate (ibidi) and cultured overnight at 37℃ in an incubator equilibrated with 95% air and 5% CO2.
- After washing once with 200μl MEM, 200 µl MEM was added.
- After 10 µl AcidSensor-labeled BSA or Mouse IgG (equivalent to 10 μg) were added to the plate, the cells were incubated at 37℃ for 2 hours in an incubator equilibrated with 95% air and 5% CO2.
- The supernatant was discarded, and the cells were washed twice with 200 µl MEM.
- The supernatant was discarded.
- MEM containing 10% FBS was added to the well, and the stained cells were observed under a confocal fluorescence microscope.
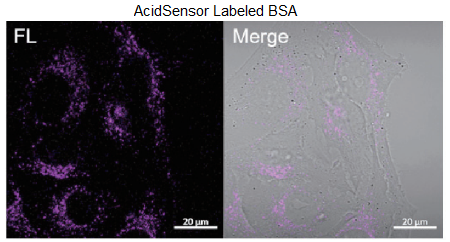
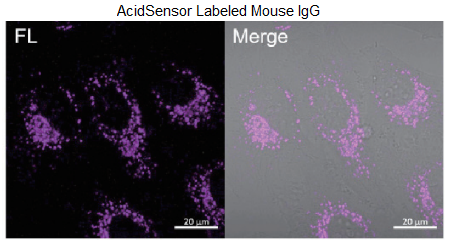
Figure 3. Obsevation of internalized proteins (BSA or Mouse IgG) via endocytosis.
Filter sets: 633 nm (Ex), 650 – 700 nm (Em)
References
- Kangqiang, Q. et al., Adv. Healthcare Mater., 2022, 11(8), 2102185.
Frequently Asked Questions / Reference
A558: AcidSensor Labeling Kit – Endocytic Internalization Assay
Revised May., 15, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

