General Information
Autophagy, a process of regulared degredation of cytoplasmic components such as proteins and organelles, has been actively studied in relation to aging and neurodegenerative diseases such as Parkinson’s disease. There is a growing need to identify whether the increase in autophagosomes is due to autophagy induction or autolysosome inhibition, and it is important to understand the autophagic flux. 1)
This kit includes autophagosome and autolysosome detection dye (DAPRed), autolysosome detection dye (DALGreen) and lysosomal acidification inhibitor (Bafilomycin A1). Autophagic Flux Assay Kit makes it possible to evaluate correctly the autophagy flux by mornitring the formation of autophagosome, fusion of lysosome and digestion of contents. 2), 3), 4), 5)
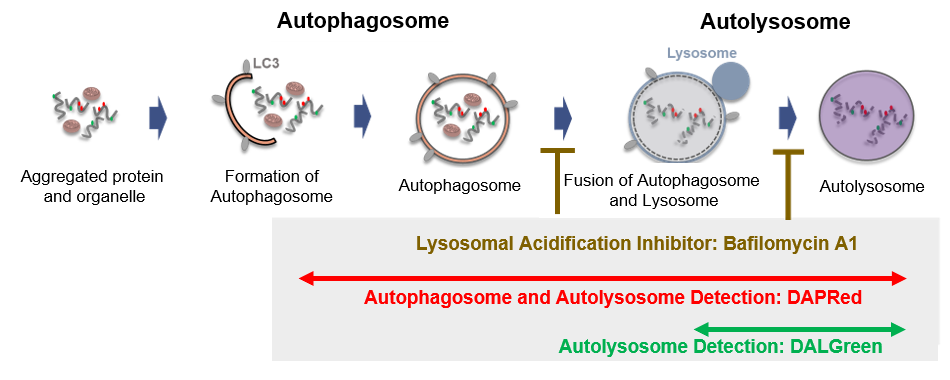
Fig 1. Autophagic flux assay by DAPRed and DALGreen
Fluorescent Property
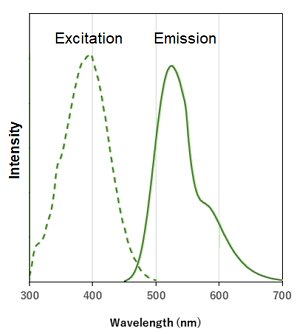
Excitation and emission spectra of DALGreen and DAPRed
|
DALGreen |
λex: 405 nm
|
DAPRed 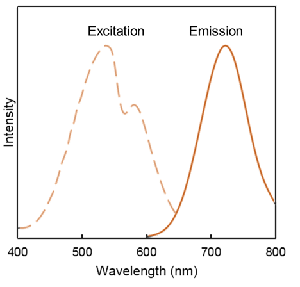 |
λex: 530 nm
|
Contents
- DALGreen 10 nmol x 1
- DAPRed 2 nmol x 1
- Bafilomycin A1 x 1
- The Bafilomycin A1 in the tube may be barely visible due to the small amount. Please handle it carefully.
Storage Condition
Store at -20°C and protect from light.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Growth medium
- Amino acid-free medium
- Micropipettes
- Microtubes
Preparation of Solution
Preparation of 1 mmol/l DALGreen DMSO stock solution
Add 10 μl of DMSO to the provided tube containing DALGreen (10 nmol), and dissolve by pipetting.
- Store the reconstituted DALGreen DMSO stock solution at -20°C until use. The solution is stable at -20°C for 1 month.
Preparation of 0.1 mmol/L DAPRed DMSO stock solution
Add 20 μl of DMSO to the provided tube containing DAPRed (2 nmol), and dissolve by pipetting.
- Store the reconstituted DAPRed DMSO stock solution at -20°C until use. The solution is stable at -20°C for 1 month.
Preparation of DALGreen/DAPRed working solution
Dilute the DALGreen DMSO stock solution with culture medium to prepare 1.0 μmol/l DALGreen working solution and dilute the DAPRed DMSO stock solution with culture medium to prepare 0.2 μmol/l DAPRed working solution.
- For example, to prepare 1 ml of the DALGreen/DAPRed working solution, mix 1 μl of DALGreen DMSO stock solution and 2 μl of DAPRed DMSO stock solution. Dilute the mixture with 1 ml of culture medium.
- DALGreen/DAPRed working solution cannot be stored. The working solution should be used up on the day it is prepared.
Preparation of Bafilomycin A1 DMSO stock solution
Add 24 μl of DMSO to a tube of Bafilomycin A1 and dissolve by pipetting.
- Store the reconstituted Bafilomycin A1 DMSO stock solution at -20°C until use. The solution is stable at -20°C for 1 month.
Preparation of Bafilomycin A1 working solution
Dilute the Bafilomycin A1 DMSO stock solution 2,000 – 10,000 times with HBSS to prepare the Bafilomycin A1 working solution.
- The optimum concentration of Bafilomycin A1 working solution varies depending on cell types. Please refer to the preliminary experiments on the product manual to determine the optimal concentration (Recommended dilution: 2,000 times).
- Bafilomycin A1 working solution cannot be stored. The working solution should be used up on the day it is prepared.
Preliminary experiments
Optimization of measurement conditions
Optimization of the measurement conditions in advance is recommended since autophagy capacity varies with cell type. When you use this product for the first time, if you are measuring different cell types, please refer to the following general protocol to optimize the staining concentration of DALGreen・DAPRed, staining concentration and incubation time of Baf. A1 to obtain the signal changes of DALGreen/DAPRed as shown below table.


- Seed cells in a dish and culture them overnight at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the culture medium and wash the cells twice with the growth medium.
- Add DALGreen/DAPRed working solution to the dish containing the cells and incubate them for 30 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the supernatant and wash the cells twice with the growth medium.
- Prepare samples under the following conditions.
- Add medium to the dish, and incubate them for 2 hours 20 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Control)
- Add amino acid-free medium to the dish, and incubate them for 2 hours 20 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Starvation)
- Add amino acid-free medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. Discard the supernatant and add Baf. A1 working solution to the dish, and incubate them for 20 minutes at 37oC in an incubator equilibrated with 95% air and 5% CO2. (Inhibition of autolysosome)
6. Observe the stained cells under a fluorescence microscope.
- Imaging analysis may be affected by the Baf. A1 dilution rate, duration of stimulation, and timing of addition. Please refer to the Q&A on the product web page for Baf. A1 stimulation.
- Please refer to the usage example on the manual and Q&A on the product web page for Baf. A1 results.
- Depending on the cell type, it may be difficult to tell the difference between starvation, Baf. A1 stimulation and control. Please optimize the appropriate concentration of DALGreen/DAPRed working solution by referring to the table above.
General Protocol
There are various agents (inducers/inhibitors) that target autophagy. Please choose the appropriate method for the experiment based on the agents. Autophagy inhibitors may affect other cellular functions by prolonged stimulation. Please consider one of the two different protocols depending on the inhibitor: "simultaneous stimulation with starvation" or "short time stimulation after starvation".
- Please refer to the FAQ on the product web page for autophagy inducer/inhibitor results.
- If the effect of the agents is unknown, please perform both protocol for autophagy induction and inhibition below.
For autophagy induction
- Seed cells in a dish and culture them at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the culture medium and wash the cells twice with the growth medium.
- Add DALGreen/DAPRed working solution to the dish containing the cells and incubate them for 30 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the supernatant and wash the cells twice with the growth medium.
- Prepare samples under the following conditions.
In the case of autophagy inducers
- Add medium to the dish, and incubate them for 24 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Control)
- Add medium containing autophagy inducer to the dish, and incubate them for 24 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Induction of autophagy)
In the case of starvation
- Add medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Control)
- Add amino acid-free medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Starvation)
6. Observe the stained cells under a fluorescence microscope.
- Please wash the cells after the stimulation, if necessary.
For autophagy inhibition
- Seed cells in a dish and culture them at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the culture medium and wash twice the cells with the growth medium.
- Add DALGreen/DAPRed working solution to the dish containing the cells and incubate them for 30 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the supernatant and wash the cells twice with the growth medium.
- Prepare samples under the following conditions.
In the case of simultaneous stimulation with starvation
- Add medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Control)
- Add amino acid-free medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Starvation)
- Add amino acid-free medium containing autophagy inhibitor to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Inhibition of autophagy)
In the case of short time stimulation after starvation
- Add medium to the dish, and incubate them for 2 hours 20 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Control)
- Add amino acid-free medium to the dish, and incubate them for 2 hours 20 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Starvation)
- Add amino acid-free medium to the dish, and incubate them for 2 hours at 37°C in an incubator equilibrated with 95% air and 5% CO2. Discard the supernatant and add amino acid-free medium containing autophagy inhibitor to the dish, and incubate them for 20 minutes at 37°C in an incubator equilibrated with 95% air and 5% CO2. (Inhibition of autophagy)
6. Observe the stained cells under a fluorescence microscope.
- Please wash the cells after the stimulation, if necessary.
- Please refer to the FAQ on the product web page for autophagy inhibitor results.
Usage Example
Analysis of autolysosome formation inhibition using Bafilomycin A1 by confocal fluorescence microscopy
- HeLa cells were seeded (1.0 x 104 cells/well) on a μ-slide 8 well plate (ibidi) and cultured overnight at 37°C in an incubator equilibrated with 95% air and 5% CO2.
- After washing twice with MEM (containing 10% fetal bovine serum), 200 μl of DALGreen/DAPRed working solution (DALGreen: 1 µmol/l, DAPRed: 0.2 µmol/l) and the cells were incubated at 37°C for 30 minutes.
- The supernatant was discarded, and the cells were washed twice with MEM (containing 10% fetal bovine serum).
- Samples were prepared under the following conditions.
- MEM (containing 10% fetal bovine serum) was added to the well, and the cells were incubated at 37°C for 2 hours 20 minutes. (Control)
- Amino acid-free medium (FUJIFILM Wako Pure Chemical Industries, Ltd., Catalogue code: 048-33575) (200 μl) was added to the well, and the cells were incubated at 37°C for 2 hours 20 minutes. (Starvation)
- Amino acid-free medium (200 μl) was added to the well, and the cells were incubated at 37°C for 2 hours. The supernatant was discarded, Bafilomycin. A1 working solution (200 μl), an inhibitor of lysosomal acidification, was added to the well, and the cells were incubated at 37°C for 20 minutes. (Inhibition of autolysosome formation)
5. The stained cells were observed under a confocal fluorescence microscope.
- Please refer to the product web page for the results of quantitative analysis of fluorescence images by image analysis software.
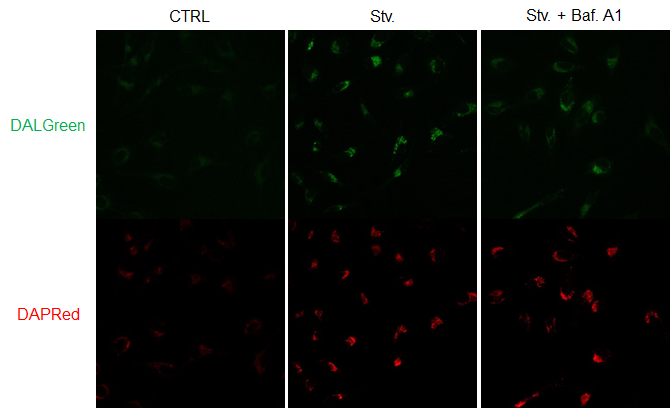
Figure 2. The effect of Bafilomycin A1 on autophagic flux
CTRL: Normal condition, Stv.: Induction of autophagy, Stv. + Baf. A1: Inhibition of autolysosome
DALGreen filter sets: 488 nm (Ex), 490–550 nm (Em)
DAPRed filter sets: 561 nm (Ex), 565–700 nm (Em)
Reference
- Xiao-jie ZHANG, et al., Acta Pharmacologica Sinica, 2013, 34, 595–599.
- H. Sakurai, et al., iScience, 2023, 26, 107218.
- Xun Chen, et al., Am J Transl Res., 2020, 12(9), 4902-4922.
- Chang-Ki Oh, et al., J Neurosci., 2022, 42(14), 3011-3024.
- Hidefumi Iwashita, et al., FEBS Lett., 2018, 592, 559-567.
Frequently Asked Questions / Reference
A562: Autophagic Flux Assay Kit
Revised Nov., 20, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.