General Information
Biofilms are biological aggregates consisting of microorganisms and extracellular polysaccharides that can exist in many environments. Bacterial contamination of medical devices, and infectious diseases such as caries and periodontal disease, are attributed to biofilms. The microorganisms in biofilms are highly resistant to antibiotics; therefore, research into medicines and food ingredients that have anti-biofilm activities is a growing area.
The Biofilm Formation Assay Kit measures the amount of biofilm formation and the anti-biofilm activity of samples. As the biofilm is formed on a peg layer on the lid of a microplate, multiple samples can easily be washed and measured at once without having to peel the biofilm off for each process.
Kit Contents
| 96 tests | |
| Crystal Violet Solution | 22 ml x 1 |
| 96-peg Lid | x 1 |
| 96-well Plate | x 10 |
Storage Conditions
Store in a cool and dark place
Required Equipment and Materials
- Microplate reader (590 nm filter)
- Incubator (37 °C)
- 20-200 μl multichannel pipette
- Ethanol
- Sterile physiological saline solution
Precautions
- This kit includes 10 sterile 96-well Plates. After opening, please keep the plates under sterile conditions until the experiments and use a new 96-well Plate in each step.
- The conditions for the formation of the biofilm vary by the species of microorganism. Please refer to table 1. Please optimize the conditions for the formation of the biofilm prior to measuring the anti-biofilm activity.
General Protocol
Measuring the amount of biofilm formation and anti-biofilm activity.
-
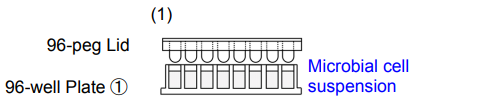
1. Add 180 μl of microbial cell suspension* to each well of 96-well Plate ①. Place the 96-peg Lid onto 96-well Plate ① and then incubate the plate at 37°C to form the biofilm on the peg.
- To measure anti-biofilm activity, add 180 μl of bacterial suspension containing.
-
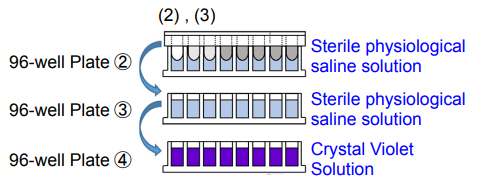
2. Add 200 μl of sterile physiological saline solution to each well of 96-well Plate ② and 96-well Plate ③. Add 200 μl of Crystal Violet Solution to each well of 96-well Plate ④.
3. Wash the 96-peg Lid from step 1 by soaking in 96-well Plates ② and ③*. Then, place the 96-peg Lid onto 96-well Plate ④. Incubate the plate at room temperature for 30 minutes.
- Please soak the lid in saline gently and avoid vigorous shaking.
-
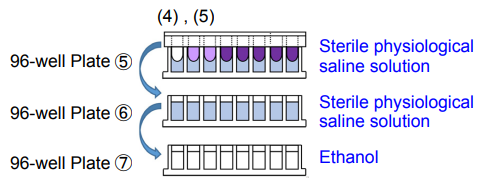
4. Add 200 μl of sterile physiological saline solution to each well of 96-well Plate ⑤ and 96-well Plate ⑥. Add 200 μl of ethanol to each well of 96-well Plate ⑦.
5. Wash the 96-peg Lid from step (3) by soaking in 96-well Plates ⑤ and ⑥*. Then, place the 96-peg Lid onto 96-well Plate ⑦. Incubate the plate at room temperature for 15 minutes.
- Please soak the lid in saline gently and avoid vigorous shaking.
-
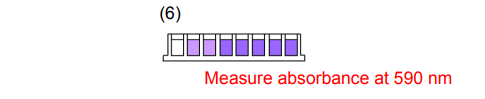
6. Remove the 96-peg Lid and measure the absorbance at 590 nm with a microplate reader.
Experimental Example
Anti-biofilm activity measurement for ε-poly-L-lysine : Staphylococcus aureus
- The microbial cell suspension was prepared at approximately 107 CFU/ml with Mueller-Hinton broth (MHB) and 180 μl of the cell suspension was added to each well of 96-well Plate ①.
- ε-Poly-L-lysine (10,000 μg/ml) was prepared with MHB medium and sterilized by filtration. The 10,000 μg/ml ε-Poly-L-lysine solution was diluted 5-fold with the 107 CFU/ml microbial cell suspension to give a 2,000 μg/ml ε-Poly-L-lysine solution. The 2,000 μg/ml ε-Poly-L-lysine solution (180 μl) was added to the leftmost well of 96-well plate ① from step 1and serially diluted to prepare a double dilution series of ε-Poly-L-lysine (180 μl in each well). The 96-peg Lid was then placed onto 96-well Plate ①, and the plate was incubated at 37°C for 24 hours.
(e.g.1000, 500, 250, 125, 62.5, 31.2, 15.6, 7.8, 3.9, 1.95, 0.98, and 0 μg/ml) - A double dilution series of ε-Poly-L-lysine, the same concentrations as step 2, was prepared with MHB medium on 96-well Plate ②.The 96-peg Lid from step (2) was then placed onto 96-well Plate ②, and the plate was incubated at 37°C for 72 hours.
- A double dilution series of ε-Poly-L-lysine, the same concentrations as step 3, was prepared with MHB medium in 96-well Plate ③. The 96-peg Lid from step (3) was then placed onto 96-well Plate ③, and the plate was incubated at 37°C for 24 hours.
- Sterile physiological saline solution (200 μl) was added to each well of 96-well Plate ④ and 96-well Plate ⑤.Crystal Violet Solution (200 μl) was added to each well of 96-well Plate ⑥.
- The 96-peg Lid from step 4 was washed by soaking in 96-well Plates ④ and ⑤. The 96-peg Lid was then placed onto 96-well Plate ⑥ and the plate was incubated at room temperature for 30 minutes.
- Sterile physiological saline solution (200 μl) was added to each well of 96-well Plate ⑦ and 96-well Plate ⑧. Ethanol (200 μl) was added to each well of 96-well Plate ⑨.
- The 96-peg Lid from step 6 was washed by soaking in 96-well Plates ⑦ and ⑧. The 96-peg Lid was then placed onto 96-well Plate ⑨ and the plate was incubated at room temperature for 15 minutes.
- The 96-peg Lid was removed and the absorbance at 590 nm was measured.
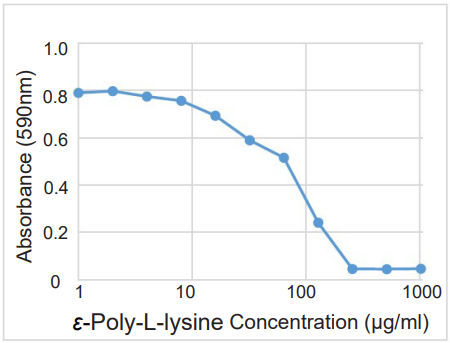
Figure. 1 Anti-biofilm activity of ε-Poly-L-lysine on S.aureus
- The MBIC (minimum biofilm inhibition concentration) of ε-Poly-L-lysine was estimated to be approx. 250 μg/ml.
| Microorganism |
Microorganism dilution medium |
Microorganism concentration [CFU/ml] |
Incubation time [h] |
Test substance dilution medium | Culture conditions |
| Pseudomonas aeruginosa | Brain heart infusion broth | 106 | 24 | MHB | Aerobic conditions |
| Escherichia coil | Brain heart infusion broth | 107 | 24+72+24 | MHB | Aerobic conditions |
| Streptococcus mutans | TSB+2%Sucrose | 107 | 24+72 | TSB+2%Sucrose | Anaerobic conditions |
| Porphyromonas gingivalis | Enriched BHI | 107 | 24+72 | Enriched BHI | Anaerobic conditions |
Enriched BHI : Enriched BHI broth (3.7% brain heart infusion, 0.5% yeast extract, and 0.05% cysteine) containing 5 μg/ml of hemin and 0.5 μg/ml of menadione.
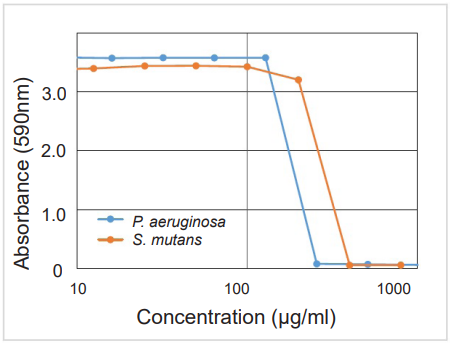
Figure. 2 Example of anti-biofilm activities
-
Test substance:P. aeruginosa / Protamine sulfate S. mutans / 4-Isopropyl-3-methylphenol
The MBIC (minimum biofilm inhibition concentration) of P. aeruginosa and S. mutans were estimated to be approx. 250 μg/ml and 400 μg/ml, respectively.
Reference
mutansT. Tsukatani, et al., J. Microbiol. Biotech. Food Sci., 2016, 6, 677-680
This product was developed through joint research within the Biotechnology and Food Research Institute, Fukuoka Industrial Technology Center.
Frequently Asked Questions / Reference
B601: Biofilm Formation Assay Kit
Revised May., 31, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.