General Information
In case quantitating an enzyme activity and a metabolite in metabolic systems such as glycolysis and citric acid cycle, corrections of measured values by the number of cells are necessary to obtain exact quantitative values (see Fig. 1). Cell Count Normalization Kit employs a nucleic acid staining dye, Hoechst 33342 which binds to nuclear DNA to emit blue fluorescence. By measuring this blue fluorescence, correction of the measured value can easily be carried out in simple steps whereas the visual cell counting method requires cumbersome procedure. Moreover, unlike the correction by protein or ATP amount, the kit requires no lysis procedure. In addition, Quenching Buffer included in the kit enables a direct measuring of fluorescence signal without any background.
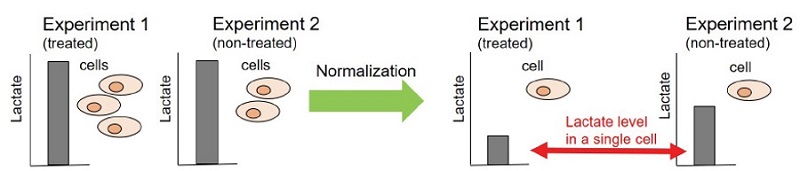
Figure 1 Necessity of normalization
Kit Contents
| 200 tests | 1000 tests | |
| Staining Solution | 50 μL x 1 | 250 μL x 1 |
| Dilution Buffer | 10 mL x 4 | 100 mL x 2 |
| Quenching Buffer | 10 mL x 2 | 100 mL x 1 |
Storage Condition
Store at 0-5 °C
Required Equipment and Materials
- Microplate reader (Ex: 350 nm, Em: 461 nm)
- 96-well black microplate (clear bottom)
- Incubator (37 °C, 5%CO2)
- Microtube (only for suspension cell)
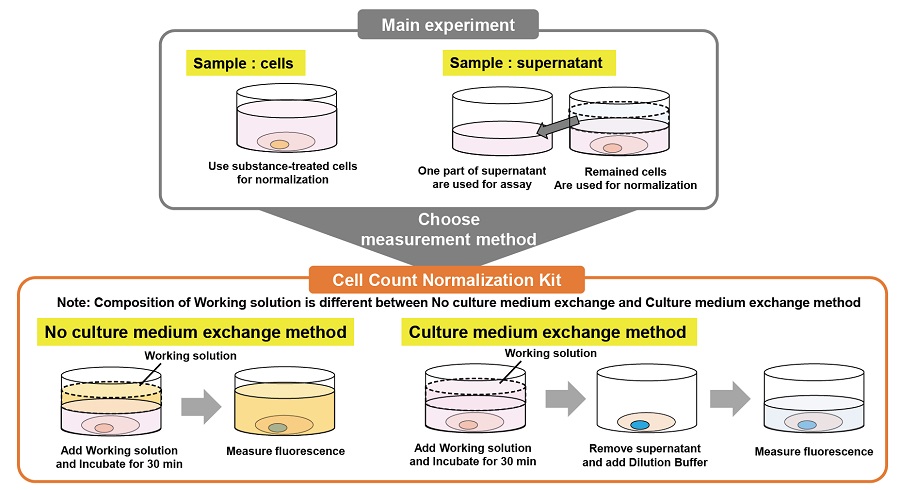
Normalization method selection guide
Refer to the following cartoons to choose a suitable experimental procedure.
-
1. No culture medium exchange method
Suitable for easily detached cells because this method does not require any washing steps.
Addition of Working solution - Incubation (30 min)
- Reading (fluorescence)
Note: Please use a plate reader that can excite and read from the bottom.
-
2. Culture medium exchange method
Both top and bottom measurements are possible.
Addition of Working solution - Incubation (30 min)
- Medium replacement
- Reading (fluorescence)
Precautions
- In order to confirm a linearity between the cell number and fluorescence signal intensity, create a calibration curve before the assay.
- Following cell numbers are recommended in case using a 96-well microplate. Adherent cells (HeLa cells): 1,000 to 10,000 cells/well, Suspension cells (Jurkat cells): 5,000 to 60,000 cells/well
- In case measuring a reagent blank, follow the experimental procedure of either No culture medium exchange method or Culture medium exchange method using wells without cells.
- In case using a fluorescent probe for the main experiment, simultaneous measurement are not recommended. Please make a normalization experiment after the main experiment.
Preparation of Solutions
Preparation of Working solution
- No culture medium exchange method Make a 500-fold dilution of Staining Solution with Quenching Buffer
- Use the Working solution within a day.
- In case using 96-well microplate, 100 μL/well of Working solution is needed for each well.
- Culture medium exchange method Make a 500-fold dilution of Staining Solution with Dilution Buffer
- Use the Working solution within a day.
- In case using 96-well microplate, 100 μL/well of Working solution is needed for each well.
General Protocol
No culture medium exchange method (only for adherent cells)
- Seed cells and incubate an appropriate time to allow cells adhere.
- Add 100 μL of Working solution to each well.
- Incubate the cells at 37 °C for 30 minutes in a 5% CO2 incubator.
- Measure fluorescence by using a microplate reader. (bottom exciting, bottom reading, Ex: 350 nm, Em: 461 nm)
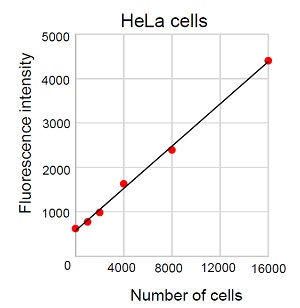
Figure 2 Calibration curve created by No culture medium exchange
Culture medium exchange method
· Adherent cells
- Seed cells and incubate an appropriate time to allow cells adhere.
- Add 100 μL of Working solution to each well.
- Incubate the cells at 37 °C for 30 minutes in a 5% CO2 incubator.
- Discard supernatant and add 100 μL of Dilution Buffer to each well.
- Measure fluorescence by using a microplate reader. (Ex: 350 nm, Em: 461 nm)
· Suspension cells
- Seed cells.
- Add 100 μL of Working solution to each well.
- Incubate the cells at 37 °C for 30 minutes in a 5% CO2 incubator.
- Collect the cells to a microtube and centrifuge at 300×g for 5 minutes.
- If you have a centrifugation system applicable for microplates, skip the step 4 and go to the step 5.
- Discard the supernatant then add 100 μL of Dilution Buffer.
- Measure fluorescence by using a microplate reader. (Ex: 350 nm, Em: 461 nm)
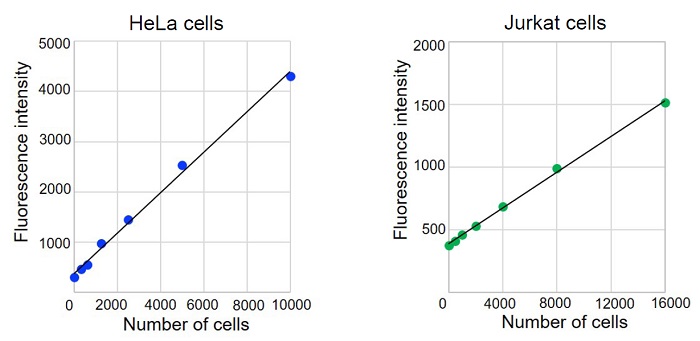
Figure 3 Calibration curves created by Culture medium exchange method
Usage Example
Evaluation of glycolysis inhibition by using 2-deoxy-D-glucose (2-DG)
(Combination use with Lactate Assay Kit-WST [L256])
- HeLa cells (5,000 cells/well) were seeded on a 96 well black microplate and cultured at 37 °C overnight in a 5% CO2 incubator
- The supernatant was aspirated and 100 μL of 2-DG solution was added to each well. The plate was incubated 37 °C overnight in a 5% CO2 incubator.
- Lactate in supernatant was assayed using Lactate Assay Kit-WST by following its technical manual.
- Assayed lactate amount (mmol/L) was normalized using Cell Count Normalization Kit (Medium exchange method).
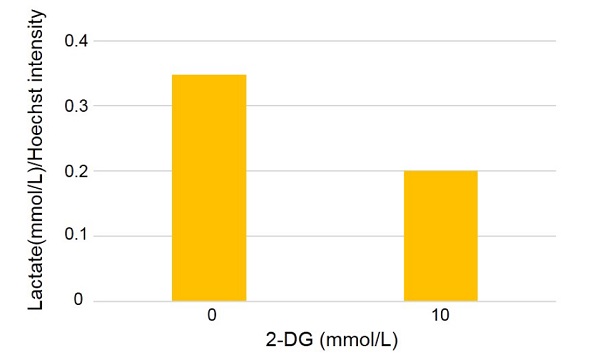
Figure 4 Amount of lactate assayed using L256 (after normalization)
Frequently Asked Questions / Reference
C544: Cell Count Normalization Kit
Revised May., 19, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

