General Information
Peroxidase Labeling Kit - NH2 is for simple and rapid preparation of peroxidase-labeled IgG for enzyme immunoassays(EIA), immunoblotting or immunostaining and peroxidase-labeled antigen for competitive EIA. NH2-Reactive Peroxidase (a component of this kit) has succinimidyl ester groups, and can easily make a covalent bond with an amino group of the target molecule without any activation process. If the target is a small molecule, the conjugate can be purified with Filtration Tube included in this kit. Filtration Tube is also used for sample IgG in removing small molecules such as sodium azide, Tris buffer and amine compounds that interfere with the assay or labeling reaction. This kit contains all of the necessary reagents for peroxidase labeling, including the storage buffer for conjugates.
Kit Contents
| NH2-Reactive Peroxidase | 1 tube |
| Reaction Buffer | 1.2 ml x 1 |
| Filtration Tube | 1 tube |
| Washing Buffer | 10 ml x 1 |
| Storage Buffer | 10 ml x 1 |
| 15 ml Tube (for counterbalance) | 1 tube |
Capacity
One sample labeling
| - Sample requirement: |
Protein (Molecular weight > 50,000; amount: 1 mg) |
Storage Condition
Store at 0-5oC. This kit is stable for 1 year at 0-5oC before opening.
|
Caution After a NH2-Reactive Peroxidase is taken out from the seal bag, keep the unused NH2-Reactive Peroxidase in the bag, seal tightly and store at -20oC. Store the other components at 0-5oC. |
Required Equipment
- 200 μl and 1 ml adjustable pipettes
- Incubator (37oC)
- Centrifuge and rotor for 15 ml tube
- Tube (> 2 ml)
Precaution
- If the target protein solution contains other proteins with molecular weight larger than 10,000 such as serum albumin or gelatin, purify the protein solution, and use the purified target proteins for peroxidase labeling, because it might interfere the labeling reaction.
- If the protein solution contains small insoluble materials, centrifuge the solution, and use the supernatant for the labeling.
- The droplets which induced from the dry inhibitor of membrane, are occasionally found inside Filtration Tube while storing the kit at 0-5oC or after returning to room temperature. This phenomenon does not affect the performance.
General Protocol -1
Labeling for Small Molecule with Amino Group
-
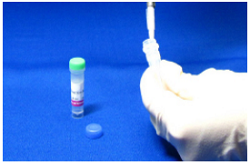
Step 1.
Prepare 0.5 ml of 1 mmol/l amine compound solutiona) with Reaction Buffer. Add this solution to NH2- Reactive Peroxidase.

Step 2.
Pipette to dissolve NH2-Reactive Peroxidase completely, and incubate the tube at 37oC for 1 h.
-
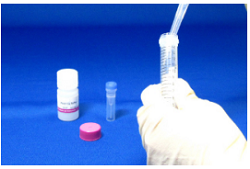
Step 3.
Add the reaction solution prepared at Step 2 and 1ml Washing Buffer to a Filtration Tube. Prepare a 15 ml Tubeb).
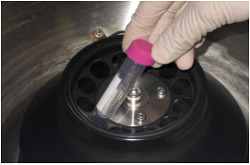
Step 4. Centrifuge at 6,000 x g for 20 min if using a fixed angle rotorc).
-
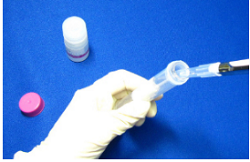
Step 5.
Discard the filtrate. Add 2 ml Washing Buffer to the Filtration Tube.
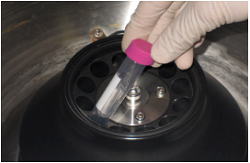
Step 6.
Centrifuge at 6,000 x g for 20 min if using a fixed angle rotor b, c). Add 2 ml Washing Buffer to the tube, and centrifuge again.
-
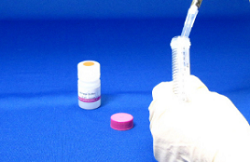
Step 7.
Add 2 ml Storage Buffer, and pipette several times to dissolve the conjugatec). Transfer the solution to a tube (not included in this kit), and store the solution at 0-5oCe).
a) If the amine compound does not dissolve in aqueous solution, dissolve it with DMSO to prepare 10 mmol/l solution, and mix 50 µl of this solution with 450 µl Reaction Buffer.
b) Measure the weight of the Filtration Tube. Prepare a same weight of 15 ml Tube with water. Use this 15 ml Tube for counter-balance.
c) Centrifuge at 4,000 x g if using a swinging bucket rotor. If more than 100 µl of the solution still remains on the membrane after the centrifugation, spin for another 10 min. If a maximum centrifugal force is less than 6,000 x g , additional spin time should be requied (ex. 2,000 x g for 50-60 min).
d) One to two target molecules should be conjugated with one peroxidase molecule.
e) We recommend using Storage Buffer to recover the conjugate. However, you can use an appropriate buffer for the downstream experiments.
General Protocol -2
Labeling for IgG
-
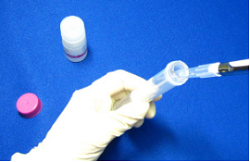
Step 1.
Add 1 ml Washing Buffer and the sample solution containing 1 mg IgGa) to a Filtration Tube. Prepare a 15 ml Tubeb).
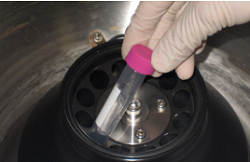
Step 2.
Pipette to mix and centrifuge at 6,000 x g for 30 min if using a fixed angle rotor b, c).
-
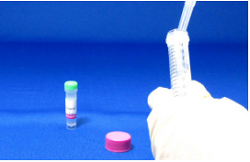
Step 3.
Add 1 ml Reaction Buffer to the Filtration Tube again.

Step 4.
Centrifuge at 6,000 x g for 30 min againc).
-
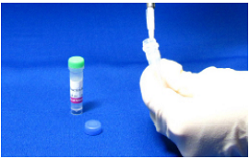
Step 5.
Add 50 µl Reaction Buffer to NH2-Reactive Peroxidase, and dissolve it with pipettingd).
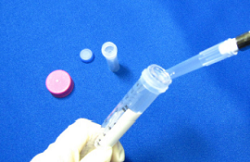
Step 6.
Add NH2-Reactive Peroxidase solution to the IgG in the Filtration Tube.
-

Step 7.
Pipette several times and Incubate the tube at 37oC for 2 h.
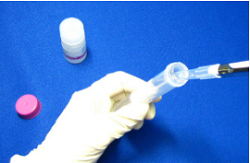
Step 8.
Add 1 ml Washing Buffer to the Filtration Tube. If the volume of the filtrate is 4 ml or more, discard the filtrate prior to go to Step 9.
-

Step 9.
Centrifuge at 6,000 x g for 30 min if using a fixed angle rotorb, c).
Step 10.
Add 2 ml Storage Buffer and pipette 10-15 times to recover the conjugatee). Transfer the solution to a microtube (not included in this kit), and store the solution at 0-5 oCf).
a) The volume of sample solution should be 3 ml or less. If the volume of sample solution is larger than 3 ml because of the low protein concentraion, repeat step 1 and 2 until the total IgG accumulation becomes 1 mg. If the volume of the fi ltrate becomes 4 ml or more during the accumulation process, discard the fi ltrate prior to going to the next centrifuge step.
b) Measure the weight of the Filtration Tube. Prepare a same weight of 15 ml Tube with water. Use this 15 ml Tube for counter-balance.
c) Centrifuge at 4,000 x g if using a swinging bucket rotor. If more than 100 µl of the solution still remains on the membrane after the centrifugation, spin for another 10 min. If a maximum centrifugal force is less than 6,000 x g, additional spin time should be requied (ex. 2,000 x g for 50 - 60 min).
d) NH2-Reactive Peroxidase is unstable in Reaction Buffer. Proceed to Step 6 immediately after the preparation of the NH2-Reactive Peroxidase solution.
e) One to three molecules of peroxidase should be introduced onto one IgG molecule. Unconjugated peroxidase should not interfere with normal immunoassays. If purification is necessary, use a gel permeation column or an affinity column for IgG.
f) We recommend using Storage Buffer to recover the conjugate. However, you can use an appropriate buffer for the downstream experiments.
Q& A
Can I use this kit to label antibody which is commercially available?
Yes. However, if antibody solution contains other proteins such as serum albumin or gelatin, labeling reaction might be interfered by that protein. Purification of the antibody solution with affinity chromatography is necessary prior to use this kit. Contact us for the purification procedure, if you need.
How long is the peroxidase labeled protein stable?
The stability depends on the protein itself. In the case of labeling for goat IgG, the labeled IgG is stable at 4oC for 2 months. However, for longer storage, add equal volume of glycerol to the sample solution and store at -20oC.
Can I use this kit for other proteins?
Yes, if the molecular weight is higher than 50,000 or lower than 5,000, and it has a reactive primary or secondary amino group. If the molecular weight is higher than 50,000, follow the labeling protocol for IgG, and use 1 mg of sample protein. If it is lower than 5,000, follow the labeling protocol for small molecules. If the molecular weight is lower than 50,000 but higher than 5,000, contact our customer service.
Can I use this kit to label oligonucleotides or peptides?
Yes, if the molecular weight is less than 5,000, and it has a reactive primary or secondary amino group. Follow the labeling protocol for small molecules.
Does NH2-Reactive Peroxidase form an oligomer during the labeling reaction?
No. Since all amino groups of NH2-Reactive Peroxidase are blocked, no oligomerization is possible.
Frequently Asked Questions / Reference
LK51: Peroxidase Labeling Kit - NH2 (for 1 mg)
Revised Nov., 29, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.