General Information
Nitric oxide (NO), a gaseous paramagnetic radical, is a very important and versatile messenger in biological systems. NO is synthesized from L-arginine by NO synthase (NOS). It has been identified as an endothelial derived relaxation factor(EDRF) and antiplatelet substance. It serves as a neurotransmitter derived from neutrophile and a cytotoxic substance from an activated macropharge. Although NO’s molecular action in the biological system is very versatile, the most important role of NO is the activation of guanylate cyclase.
The Griess assay is one of the most popular and simplest methods used to detect the NO concentration. The Griess assay machanism is summarized as the azo coupling between diazonium species, which are produced from sulfanilamide with NO2, and naphthylethylenediamine(Fig.1). The NO2/NO3 Assay Kit-CII contains these dyes, Nitrate Reductase, Enzyme Co-factors, Buffer Solution and NO2, NO3 Solutions as standards. Therefore, total NO metabolites (Scheme 1) are easily detectable using this kit. The suitable NO2 detection range is from 10 to 100 μmol/l.
-
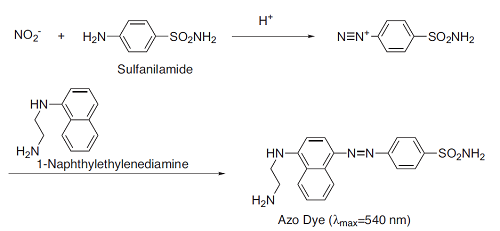
Fig. 1 Coloring reaction scheme of NO2- detection
-
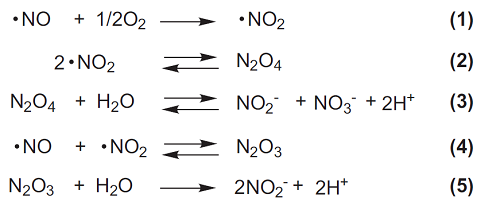
Scheme 1 NO reaction and its metabolites
Contents of The Kit
| NaNO2 Standard Solution (100 μmol/l) | x 1 |
| NaNO3 Standard Solution (100 μmol/l) | x 1 |
| Griess Reagent A | x 1 |
| Griees Reagent B | x 1 |
| Buffer Solution (20 mmol/l, pH 7.6) | x 1 |
| Nitrate Reductase (Dissolve with 1.2 ml of Buffer Solution prior to use) | x 1 |
| Enzyme Co-factors (Dissolve with 1.2 ml of Buffer Solution prior to use) | x 1 |
Storage
Store the kit at 0-5°C. The solutions of Nitate Reductase and Enzyme Co-factors, should be stored at 0-5°C and used up within 2 weeks.
Precaution
- This kit contains glass vials. Please handle them carefully.
- Both Nitrate Reductase and Enzyme Co-factors solutions should be kept in ice bath during your experiment.
- NO2 and NO3 Standard Solution should be used up within 2 months once the vial is opened.
- This kit is able to determine nitrate and nitrite when the concentration range from 10 to 100 μmol/l. Dilute a sample if it contains high concentrations of nitrate and nitrite. The total volume of sample should be no less than 80 μl. Add Buffer Solution to a sample as necessary.
- Prepare a calibration curve for each experiment. NO2 calibration curve should not be used to determine the NO3 concentration, and NO3 calibration curve should not be used to determine the NO2 concentration.
- Do not mix Nitrate Reductase with Enzyme Co-factors solution prior to use.
- Since inside of each vial of Nitrate Reductase and the Enzyme Co-factors is under reduced pressure, add 1.2 ml Buffer Solution into the vial with a syringe in order to avoid the dispersal of the powder. Please do not open the rubber septum at the beginning.
Required Equipment and Materials
- Microplate reader (530 - 580 nm filter: Optimum at 540 nm)
- 96-well microplate
- 10 μl, 100-200 μl pipettes, multichannel pipettes
General Protocol for 96-well Microplate Assay
- Add NaNO2 Standard Solution and Buffer Solution to each well as follows.
Well NaNO2 Standard Solution(μl) Buffer
Solution(μl )Final conc. of NO2(μmol/l ) A1 0 80 0 B1 20 60 25 C1 40 40 50 D1 80 0 100 - Add 20 μl of Buffer Solution to each well (total volume is 100 μl/well).
- Add 50 μl of Griess Reagent A to each well, and mix, incubate the plate for 5 minutes at room temperature.
- Add 50 μl of Griess Ragent B to each well, and mix. After 10 minutes of incubation at room temperature, measure the absorbance of each well at 540 nm with a microplate reader.
- Determine the absorbance of each well with subtracting blank(well A1) .
- Plot the concentrations of NaNO2 solution as X-axis and the absorbance values as Y-axis to prepare a calibration curve.
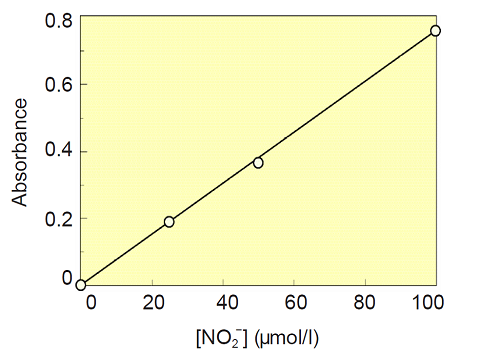
Fig.2 Calibration Curve of Nitrite Solution
Preparation of NO2- + NO3- Calibration Curve
- Add NaNO3 Standard Solution and Buffer Solution to each well as follows.
Well NaNO3 Standard Solution(μl) Buffer
Solution(μl )Final conc. of NO3(μmol/l ) E1 0 80 0 F1 20 60 25 G1 40 40 50 H1 80 0 100 - Add 10 μl of the Nitrate Reductase solution and 10 μl of the Enzyme Co-factors solution to each well, and mix.
- Incubate the plate at room temperature for 2 hours.
- Add 50 μl of Griess Reagent A to each well, and mix. Then, incubate the plate for 5 minutes at room temparature.
- Add 50 μl of Griess Ragent B to each well, and mix. After 10 minutes of incubation at room temperature, measure the absorbance of each well at 540 nm with a microplate reader.
- Determine the absorbance of each well with subtracting blank(well E1) .
- Plot the concentrations of NaNO3 solution as X-axis and the absorbance values as Y-axis to prepare the calibration curve.
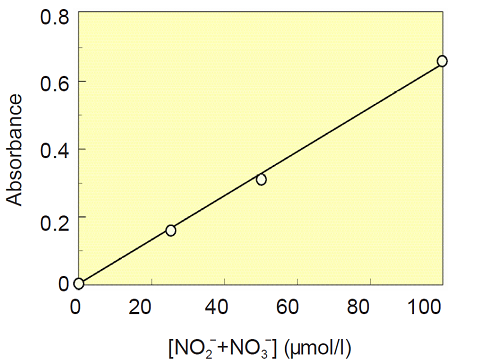
Fig.3 Calibration Curve of [NO2- + NO3-]
Preparation of Sample Solution
Cell or tissue culture medium:
Centrifuge the medium at 1000xg for 15minutes, and use the supernatant as a sample solution.
A cell culture medium that contains NO3- as a component (such as RPMI1640) cannot be used suitable for [NO2- + NO3- ] detection.However, NO2- in the medium can be determined using this kit.
Serum:
Serum or plasma sample should be treated with Amicon Ultra-4 Centrifugal Filter Unit with Ultracel-10 membrane
(7000xg, 4oC, 20minutes) to remove hemoglobin and protein.
Determination of NO2-Concentration in Sample Solution
- Add 80 μl of a sample solution to a well.
- Add 20 μl of Buffer Solution to each well (total volume is 100 μl/well).
- Add 50 μl of Griess Reagent A to each well, and mix. Then, incubate the plate for 5minutes at room temperature.
- Add 50 μl of Griess Reagent B to each well, and mix. After incubation for 10minutes at room temperature, measure the absorbance at 540 nm with a microplate reader.
- Subtract the absorbance of the blank solution (well A1) from the absorbance of the well.
- Determine the concentration of nitrite in the sample solution using the calibration curve.
Determination of NO2- + NO3-- Concentration in Sample Solution
- Add 80 μl of a sample solution to a well.
- Add 10 μl of the Nitrate Reductase solution and 10 μl of the Enzyme Co-factors solution to each well, and mix.
- Incubate the plate at room temparature for 2 hours.
- Add 50 μl of Griess Reagent A to each well, and mix. Then, incubate the plate for 5minutes at room temperature.
- Add 50 μl of Griess Reagent B to each well, and mix. After 10minutes of incubation at room temperature, measure the absorbance at 540 nm with a microplate reader.
- Subtract the absorbance of the blank solution (well E1) from the absorbance of the well.
- Determine the concentration of [NO2- + NO3-] in the sample solution using the calibration curve.
Determination of Nitrate Concentration in Sample Solution
Nitrate concentration can be obtained by the following equation.
[NO3-] = [NO2- + NO3-] - [NO2-]
References
- S. Archer, FASEB. J., 1993, 7, 349.
- W. R. Tracy, J. Linden, M. J. Prach, R. A. Johns, J. Pharmacol. Exp. Ther., 1990, 252, 922.
- J. S. Pollock, U. Forstermann, J. A. Mitchell, T. D. Warner, H. H. H. Schmidt, M. Nakane, F. Murad, Proc. Natl. Acad. Sci. U.S.A., 1991, 88, 10480.
- H. H. H. Schmidt, T. D. Warner, M. Nakane, U. Forstermann, F. Murad, Mol. Pharmacol., 1992, 41, 615.
Frequently Asked Questions / Reference
NK05: NO2 / NO3 Assay Kit - C II (Colorimetric) - Griess Reagent Kit -
Revised Dec., 05, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

