-Bacstain- Bacterial Viability Detection Kit - CFDA/PI
Bacterial Staining
-
Product codeBS10 -Bacstain- Bacterial Viability Detection Kit - CFDA/PI
| Unit size | Price | Item Code |
|---|---|---|
| 1.0 set x 1 | $417.00 | BS10-10 |
| 1.0 set x 1 | CFDA Solution PI Solution |
375 μl × 4 25 μl × 4 |
|---|
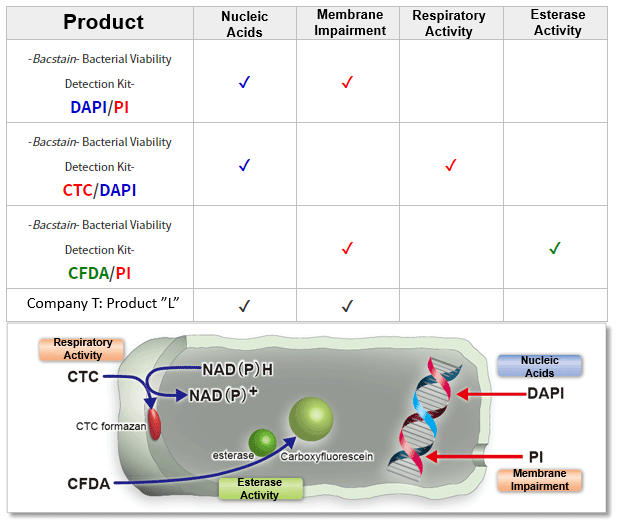
Detection Principle
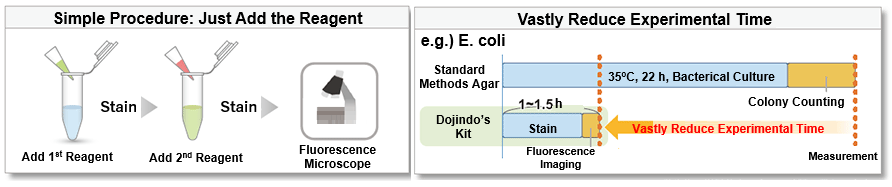
-Bacstain- Bacterial Viability Detection Kits are a series of products for fluorescent double-staining of bacteria. By combining different types of fluorescent stain, staining images can be acquired for each index (membrane impairment, respiratory activity, and esterase activity). Bacterial viability is generally assessed by colony formation in the nutrient-agar medium. However, this requires a long culturing time, and it is hard to recognize the growth of viable but nonculturable (VNC) bacteria. However, fluorescent staining does not require bacterial culture and enables viability assessment by rapid and simple protocols.
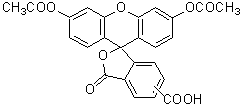
-Bacstain- Bacterial Viability Detection Kit – CFDA/PI uses esterase activity of bacteria and bacterial membrane damage as an index. CFDA is hydrolyzed to carboxyfluorescein―which is fluorescent―by intracellular esterase. PI is a parallel intercalator into the DNA double helix that stains nucleic acids; it passes only through damaged bacterial membranes. Thus, this kit can be used to measure the ratio of bacteria with esterase activity to membrane-damaged bacteria on the analysis of the fluorescent images from each stain.
Simple Procedure
Manual
Technical info
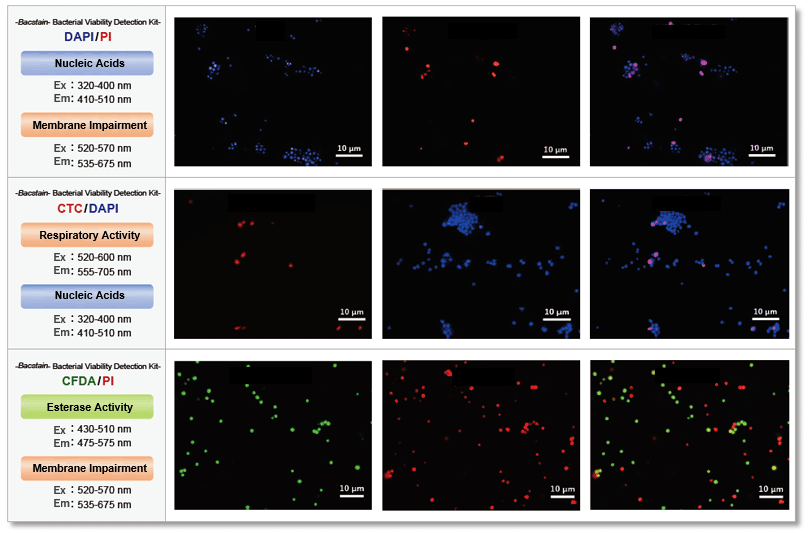
Quantitative analysis
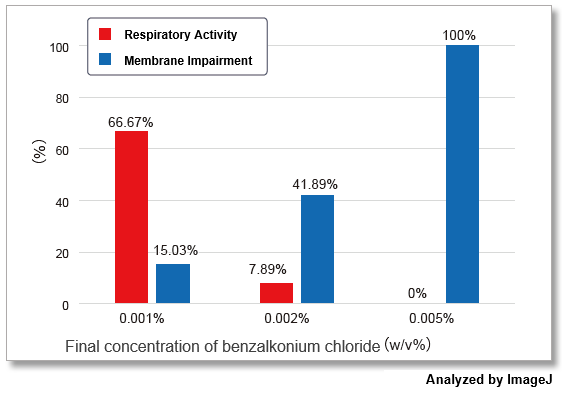
-Bacstain- Bacterial Viability Detection Kit – DAPI/PI (BS08) and –CTC/DAPI (BS09) were used to evaluate the effect of benzalkonium chloride on S. aureus (Gram-positive bacteria) by fluorescent imaging. The images were quantified by ImageJ and show the correlation with drug concentration.
The results showed that the effects of benzalkonium chloride on the respiratory activity and membrane damage of S. aureus varied greatly depending on the indicator detected.
The evaluation of multiple indicators will contribute to improving the reliability of drug efficacy measurements by providing a multidimensional view of the activity of the bacterium that would be missed by a single indicator.
Handling and storage condition
| -20°C, Protect from light | |
|
Danger / harmful symbol mark |

|
|---|---|