-Cellstain- Calcein-AM solution
Live Cell Staining
-
Product codeC396 -Cellstain- Calcein-AM solution
-
CAS No.148504-34-1(Calcein-AM)
-
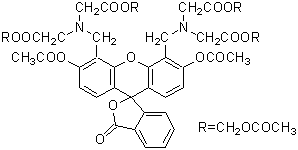
Chemical name3',6'-Di(O-acetyl)-4',5'-bis[N,N-bis(carboxymethyl)aminomethyl]fluorescein, tetraacetoxymethyl ester, DMSO solution
-
MWC46H46N2O23=994.86
| Unit size | Price | Item Code |
|---|---|---|
| 1 ml | $222.00 | C396-10 |
1 mg/mL DMSO solution
Description
Product Description
Calcein-AM readily passes through the cell membrane of viable cells because of its enhanced hydrophobicity compared to Calcein. After Calcein-AM permeates into the cytoplasm, it is hydrolyzed by esterases to Calcein, which remains inside the cell (Fig. 1). Among other reagents, including BCECF-AM and Carboxy-fluorescein diacetate, Calcein-AM is the most suitable fluorescent probe for staining viable cells because of its low cytotoxicity. Calcein does not inhibit any cellular functions such as proliferation or chemotaxis of lymophocyte. In addition, viability assays using Calcein are reliable and correlate well with the standard 51Cr-release assay. The excitation and emission wavelengths of calcein are 490 nm and 515 nm, respectively
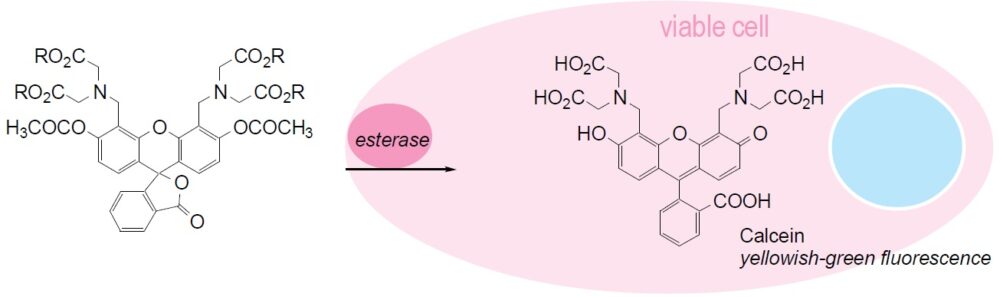
Fig. 1 Cell staining mechanism
Technical info
Staining Procedure
1.Prepare 1 mM Calcein-AM solution with DMSO and dilute to prepare 1-50 μM Calcein-AM solution with PBS.a)
2.Add Calcein-AM solution with 1/10 of the volume of cell culture medium to the cell culture.b)
3.Incubate the cell at 37ºC for 15-30 min.
4.Wash cells twice with PBS or an appropriate buffer.
5.Observe the cells under a fluorescence microscope with 490 nm excitation and 515 nm emission filters.
a) If the Calcein-AM has difficulty loading into cells, use a detergent such as Pluronic F127.
b) Or you may replace the culture medium with 1/10 concentration of Calcein-AM buffer solution.
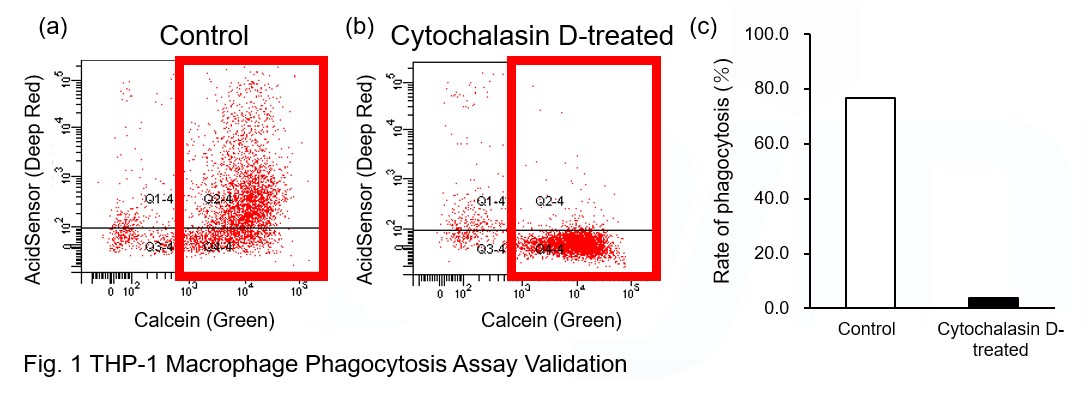
Experimental Example: Phagocytosis assay of labeled apoptotic cells in THP-1 cells
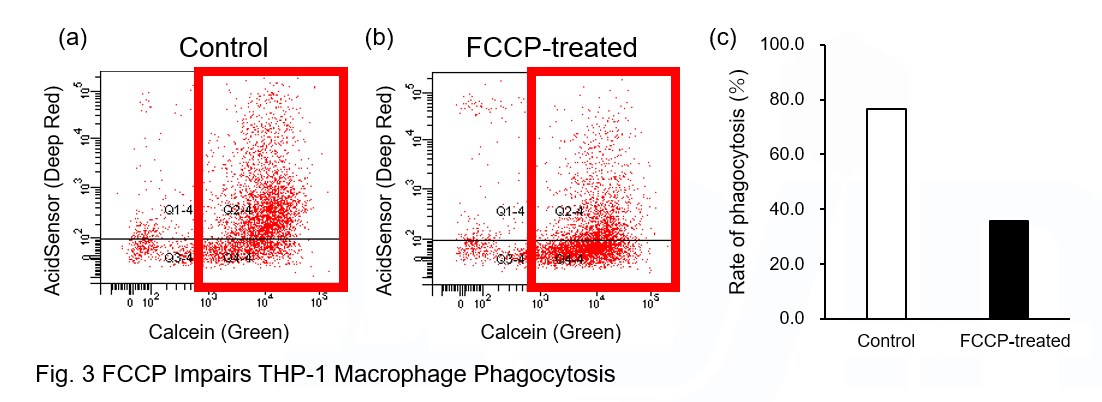
AcidSensor-labeled substances are taken up by cells and their fluorescence increases when they reach acidic organelles such as lysosomes. Taking advantage of this property, we evaluate the phagocytic activity of apoptotic cells by co-culturing AcidSensor-labeled apoptotic cells with Calcein-labeled THP-1 macrophages. As a result, Calcein (Green) / AcidSensor (Deep red) double-positive cells, indicating THP-1 macrophages phagocytosing apoptotic cells, were observed by flow cytometry (Fig. 1a). Furthermore, when the phagocytosis of THP-1 macrophages was inhibited by Cytochalasin D, the percentage of double-positive cells decreased (Fig. 1b and 1c), confirming that the assay system can accurately evaluate phagocytosis.
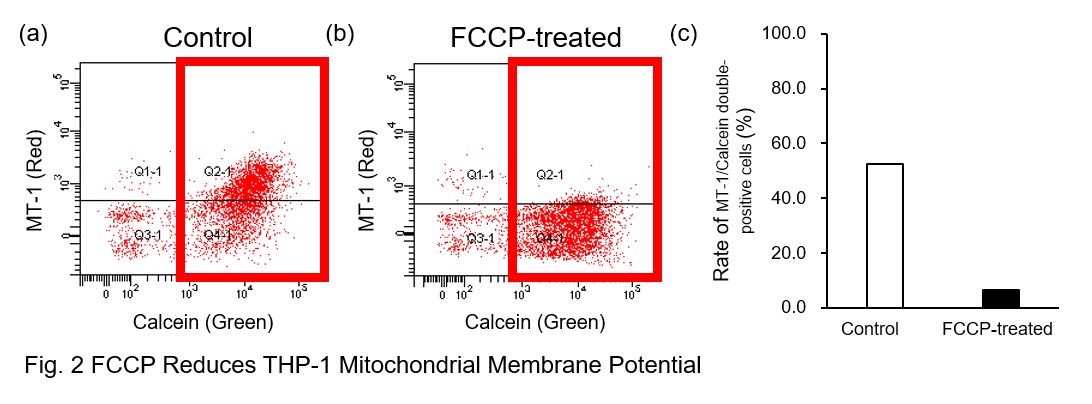
A recent report reveals that inhibition of mitochondrial function induces a switch to glycolysis and reduces phagocytosis in cultured microglia, resident macrophages in the central nervous system*. To replicate this result, phagocytosis assays were performed using mitochondria-inhibited THP-1 macrophages. The results show that FCCP, a potent uncoupler of oxidative phosphorylation in mitochondria, decreases mitochondrial membrane potential (MT-1, Red) of THP-1 macrophages (Fig. 2) and reduces phagocytosis (Fig. 3).
*Lauren H. Fairley, et al., PNAS (2023)
Products in Use
① AcidSensor Labeling Kit – Endocytic Internalization Assay [code: A558]
② -Cellstain- Calcein-AM solution [code: C396]
③ MT-1 MitoMP Detection Kit [code: MT13]
[Experimental Procedure]
Preparation of AcidSensor-labeled apoptotic cells (day before assay)
1. Add 10 μl of DMSO to NH2-Reactive AcidSensor and dissolve. 5 μl NH2-Reactive AcidSensor solution was added to 5 ml HBSS to make the Working solution (1000-fold dilution).
2. Wash Jurkat cells twice with HBSS.
3. Jurkat cells (5×107 cells) were transferred to the tube and the supernatant was removed after centrifugation.
4. Add the Working solution to the tube with Jurkat cells (5×107 cells) and suspend.
5. Incubate at 37°C for 30 minutes for labeling with AcidSensor.
6. After labeling, the cells were washed twice with HBSS.
7. AcidSensor-labeled Jurkat cells were suspended in RPMI medium with 10% FBS added with 0.5 μM staurosporine.
8. Apoptosis was induced by o/n culture in an incubator (37°C, 5% CO2) for 18 hours.
9. After induction of apoptosis, the cells were washed with a culture medium and used for the phagocytosis assay.
Phagocytosis assay using THP-1 macrophages
1. To differentiate THP-1 cells into macrophages, THP-1 cells were seeded into 6 well plates at 1x106 cells/well and incubated with 100 nM PMA for 3 days in an incubator.
2. After washing THP-1 macrophages twice with HBSS, Calcein-AM (Code: C396, 0.5 μg/ml) and MT-1 (Code: MT13, 1000-fold dilution) included HBSS solution was added to the wells and incubated in the incubator for 30 minutes.
3. Wash twice with a culture medium.
4. The cells were incubated with 10 μM Cytochalasin D for 1 hour or 5 μM FCCP for 30 minutes in the incubator.
5. After twice washing with culture medium, AcidSensor-labeled apoptotic cells (3x106 cells/well) were added to the well with or without Cytochalasin D or FCCP. The cells were incubated in the incubator for 4 hours.
6. Wash twice with HBSS.
7. Add 500 μl Imaging buffer solution (Code: MT13) and collect THP-1 macrophages from plates with a cell scraper.
8. Cell suspensions were analyzed by flow cytometry.
Data
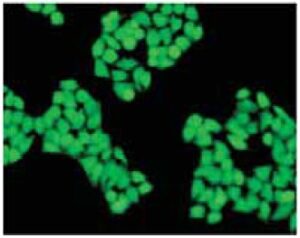
Fig. 2 Cell staining with Calcein-AM
Cell type: HeLa
References
1. K. McGinnes, et al., A Fluorescence NK Assay Using Flow Cytometry. J Immunol Methods. 1986;86:7-15.
2. S. J. Morris, Real-time Multi-wavelength Fluorescence Imaging of Living Cells. BioTechniques. 1990;8:296-308.
3. S. A. Weston, et al., New Fluorescent Dyes for Lymphocyte Migration Studies Analysis by Flow Cytometry and Fluorescent Microscopy. J Immunol Methods. 1990;133:87-97.
4. D. M. Callewaert, et al., Characterization of Effector-Target Conjugates for Cloned Human Natural Killer and Human Lymphokine Activated Killer Cells by Flow Cytometry. Cytometry. 1991;12:666-676.
5. H. Xie, et al., Intercellular Communication Through Gap Junctions Is Reduced in Senescent Cell. Biophys J. 1992;62:45-47.
6. S. A. Weston, et al., Calcein: a Novel Marker for Lymphocytes Which Enter Lymph Nodes. Cytometry. 1992;13:739-749.
7. X. M. Wang, et al., A New Microcellular Cytotoxicity Test Based on Calcein AM Release. Hum Immunol. 1993;37:264-270.
8. N. G. Papadopoulos, et al., An Improved Fluorescence Assay for the Determination of Lymphocyte-Mediated Cytotoxicity Using Flow Cytometry. J Immunol Methods. 1994;177:101-111. 9. L. S. D. Clerck, et al., Use of Fluo
rescent Dyes in the Determination of Adherence of Human Leucocytes to Endothelial Cells and the Effects of Fluorochromes on Cellular Function. J Immunol Methods. 1994;172:115-124.
10. H. Ohata, et al., Confocal Imaging Analysis of ATP-Induced Ca2+ Response in Individual Endothelial Cells of the Artery in Situ. Am J Physiol. 1997;272:C1980-C1987.
Handling and storage condition
| Appearance: | Colorless liquid |
|---|---|
| Dye content: | To pass test |
| -20°C, Protect from light | |
|
Danger / harmful symbol mark |

|
|---|---|