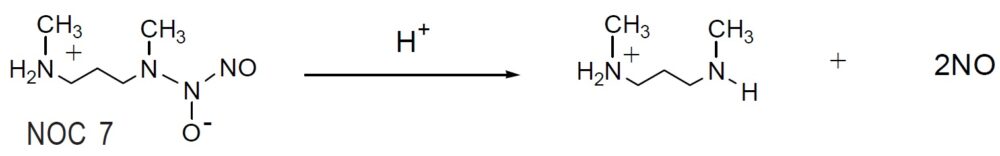
NOC 7

NO Detection
-
Product codeN377 NOC 7
-
CAS No.146724-84-7
-
Chemical name1-Hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene
-
MWC5H14N4O2=162.19
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | $166.00 | N377-10 |
| 50 mg | $598.00 | N377-12 |
Reaction of NO release
Product Description of NOC Compounds
NOCs are stable NO-amine complexes that spontaneously release NO, without cofactors, under physiological conditions. The rate of NO release depends on the chemical structure of NOC. The mechanism of spontaneous NO generation by NOCs is very simple compared to other classical NO donors, such as nitroglycerin and nitropurusside, and the by-products do not interfere with cell activities. A single NOC molecule releases two NO molecules (as indicated in the reaction scheme); the release rate of the second NO molecule is very slow. NOCs can be used to add controlled amounts of pure NO to experimental systems at controlled rates with minimal side effects. The amount of NO released can be easily manipulated by altering the concentration and selection of NOC reagents. Dojindo offers four different NOCs (NOC 5, 7, 12, and 18) with different halflifes. Stock solutions of NOC prepared in alkaline solutions, such as aqueous NaOH, are relatively stable. However,the NOC stock solution should be used within one day because it degrades about 5% per day, even at -20ºC. The release of NO begins immediately after adding the stock solution to a sample solution.
Technical info
1. Prepare 10 mM NOC stock solution using 0.1 M NaOH. Since the NOC stock solution is not stable, keep it on an ice bath and use it in one day.
2. Add an appropriate volume of the NOC stock solution to the sample solution in which NO is to be released. To maintain the pH of the sample solution, the volume of the NOC stock solution should not exceed 1/50 of the sample volume. The sample solution should have sufficient buffering action. NO will be released immediately after the addition of the NOC stock solution.
Table 1 pH Dependency of NO Release at 37oC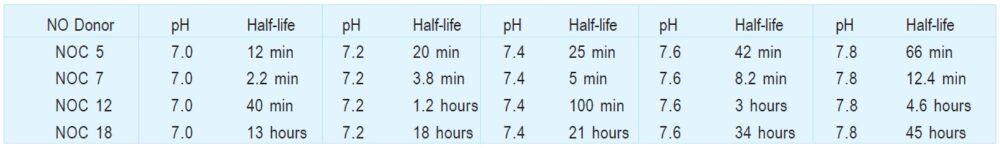
References
1)J. A. Hrabie, J. R. Klose, D. A. Wink and L. K. Keefer, "New Nitric Oxide-releasing Zwitterions Derived from Polyamines.", J. Org. Chem., 1993, 58, 1472.
2) K. Hayashi, N. Noguchi and E. Niki, "Action of Nitric Oxide as a Antioxidant Against Oxidation of Soybean Phosphatidylcholine Liposomal Membrane", FEBS Lett., 1995,370, 37.(Noc 12)
3) S. Shibuta, T. Mashimo, A. Ohara, P. Zhang and I. Yoshiya, "Intracerebroventricular Administration of a Nitric Oxide-releasing Compound, NOC-18, Produces Thermal Hyperalgesia in Rats", Neurosci. Lett., 1995, 187, 103. (NOC18)
4) N. Yamanaka, O. Oda and S. Nagao, "Nitric Oxide Released from Zwitterionic Polyamine/NO Adducts Inhibits Cu2+-induced Low Density Lipoprotein Oxidation", FEBS Lett., 1996, 398 , 53.
5) D. Berendji, V. K-Bachofen, K. L. Meyer, O. Grapenthin, H. Weber, V. Wahn and K.-D Kroncke, "Nitric Oxide Mediates Intracytoplasmic and Intranuclear Zinc Release", FEBS Lett., 1997, 405, 37.
6) T. Ohnishi, T. Ishizaki, F. Sasaki, S. Ameshima, T. Nakai, S. Miyabo and S. Matsukawa, "The Effect of Cu2+ on Rat Pulmonary Arterial Rings", Eur. J. Pharmacol., 1997, 319, 49. (Noc 7)
7) Y. Adachi, K. Hashimoto, N. Ono, M. Yoshida, M. Suzuki-Kusaba, H. Hisa and S. Satoh, "Renal Effect of a Nitric Oxide Donor, NOC 7, in Anethetized Rabbits", Eur. J. Pharmacol., 1997, 324, 223. (Noc 7)
8) Y. Minamiyama, S. Takemura and M. Inoue, "Effect of Thiol Status on Nitric Oxide Metabolism in the Circulation", Arch. Biochem. Biophys., 1997, 341(1), 186.
1. K. Hayashi, et al., Action of Nitric Oxide as a Antioxidant Against Oxidation of Soybean Phosphatidylcholine Liposomal Membrane. FEBS Lett. 1995;370:37-40. (NOC 12)
2. S. Shibuta, et al., Intracerebroventricular Administration of a Nitric Oxide-releasing Compound, NOC-18, Produces Thermal Hyperalgesia in Rats. Neurosci Lett. 1995;187:103-106. (NOC 18)
3. S. Shibuta, et al., A new nitric oxide donor, NOC-18, exhibits a nociceptive effect in the rat formalin model. J Neurol Sci. 1996;141:1-5. (NOC 18)
4. N. Yamanaka, et al., Nitric Oxide Released from Zwitterionic Polyamine/NO Adducts Inhibits Cu2+-induced Low Density Lipoprotein Oxidation. FEBS Lett. 1996;398:53-56. (NOC 5, NOC 7)
5. D. Berendji, et al., Nitric Oxide Mediates Intracytoplasmic and Intranuclear Zinc Release. FEBS Lett. 1997;405:37-41.
6. T. Ohnishi, et al., The Effect of Cu2+ on Rat Pulmonary Arterial Rings. Eur J Pharmacol. 1997;319:49-55. (NOC 7)
7. Y. Adachi, et al., Renal Effect of a Nitric Oxide Donor, NOC 7, in Anethetized Rabbits. Eur J Pharmacol. 1997;324:223-226. (NOC 7)
8. Y. Minamiyama, et al., Effect of Thiol Status on Nitric Oxide Metabolism in the Circulation. Arch Biochem Biophys. 1997;341:186-192. (NOC 7)
Q & A
-
Q
How do I prepare a stock solution?
-
A
Prepare 10-50 mM NOC solution with 0.1 M NaOH solution. Then add enough NOC solution to the cell culture to obtain a suitable concentration of NOC in cell culture. If the pH of the culture solution changes, use higher concentration of NOC.
-
Q
What is the solubility of the NOC compounds?
-
A
NOC 5: 40 mg per 100 ml 0.1 M NaOH (2.2 M NOC 5)
NOC 7: 70 mg per 100 ml 0.1 M NaOH (4.3 M NOC 7)
NOC 12: 27 mg per 100 ml 0.1 M NaOH (1.5 M NOC 12)
NOC 18: 20 mg per 100 ml 0.1 M NaOH (1.2 M NOC 18)
-
Q
Is the stock solution stable?
-
A
The stock solution will lose 5% of its NOC activity per day, even when stored at -20oC. Please prepare fresh solution prior to use and keep the solution on an ice bath during the experiment. VI-1. Nitric Oxide Research: NO Donors
-
Q
How is the half-life of NOC determined?
-
A
Prepare 20 mM NOC stock solution with 0.1 M NaOH. Warm PBS at 37ºC. Add 100 ml NOC solution to 1.9 ml PBS. Using a UV spectrophotometer, immediately start measuring its absorbance at the maximum wavelength of the NOC. Continue measuring until no further spectra changes are observed.
-
Q
Can I use NOC for in vivo experiments?
-
A
Yes. Please review the papers by Shibata and colleagues (1995, 1996).
-
Q
Is the amount of NO released in vitro the same as in vivo?
-
A
The amount of NO released in the solution should be the same if the pH and temperature are the same. However, the activity of NO may be different in vivo because of other reactive components such as thiol compounds and heme.
Handling and storage condition
| Appearance: | White powder |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| -20°C, Protect from light |










