|
Cell death and the dysfunction of autophagy and lysosomes are closely linked in maintaining cellular health. When autophagy is impaired, cells cannot effectively degrade and recycle damaged components, leading to the accumulation of toxic materials and cellular stress. Lysosomal dysfunction exacerbates this problem, as these organelles are essential for the degradation of autophagosomal contents. The resulting cellular damage and stress can trigger cell death pathways that contribute to various diseases, including neurodegenerative disorders and cancer.
|
-
Copper-dependent autophagic degradation of GPX4 drives ferroptosis
Click here for the original article: Qian Xue, et. al., Autophagy, 2023.
Point of Interest
- Copper chelators inhibit ferroptosis but do not inhibit other types of cell death, such as apoptosis, necroptosis, and alkaliptosis.
- On the other hand, exogenous copper directly binds to cysteines of GPX4, leading to ubiquitination and aggregation of GPX4.
- Ubiquitinated and aggregated GPX4 induces autophagy via the Tax1 binding protein and is subsequently degraded.
- Copper enhances ferroptosis and suppresses tumor progression in a mouse model.
-
Scd1 and monounsaturated lipids are required for autophagy and survival of adipocytes
Click here for the original article: Hiroyuki Mori, et. al., Molecular Metabolism, 2024.
Point of Interest
- Scd1, an enzyme that converts saturated fatty acids to monounsaturated fatty acids, is expressed much higher in lipogenic tissues and adipocytes than in other cell types.
- Scd1-deficiency leads to lysosomal dysfunction and ultimately to the accumulation of non-acidic autophagosomes.
- This results in vacuole accumulation and eventual autophagy-dependent cell death.
- Inhibition of autophagosome formation or supplementation with monounsaturated fatty acids maintains the vitality of Scd1-deficient adipocytes.
-
Glucose starvation causes ferroptosis-mediated lysosomal dysfunction
Click here for the original article: Kenji Miki, et. al., iScience, 2024.
Point of Interest
- Glucose starvation causes an increase in autophagic proteins but a decrease in lysosomal protein expression.
- This result leads to dysfunction of lysosomes and accumulation of damaged lysosomes.
- Lysosomal dysfunction is mainly caused by several enzymes of the glycolytic and NAD pathways, including ALDOA, GAPDH, NAMPT, and PGK1, which do not function effectively under glucose starvation.
- Ferroptosis occurs via iron accumulation due to dysfunction of DMT1, which excretes iron from lysosomes.
|
|
Related Techniques
|
|
|
- Autophagy detection
- DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
|
|
- Ferrous ion (Fe2+) detection
- FerroOrange, Mito-FerroGreen
|
- Lipid droplet detection
- Lipid Droplet Assay Kit - Blue / Deep Red
|
- Mitophagy detection dye
- GSSG/GSH Quantification Kit
|
- Cellular senescence detection
- SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples.
|
- Glycolysis/Oxidative phosphorylation Assay
- Extracellular OCR Plate Assay Kit, Glycolysis/OXPHOS Assay Kit
|
- NAD(H) and NADP(H) redox couples assay
- NAD/NADH and NADP/NADPH Assay Kit
|
|
Related Applications
|
Tracing autophagosome to autolysosome in live cells
Nampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021)
-
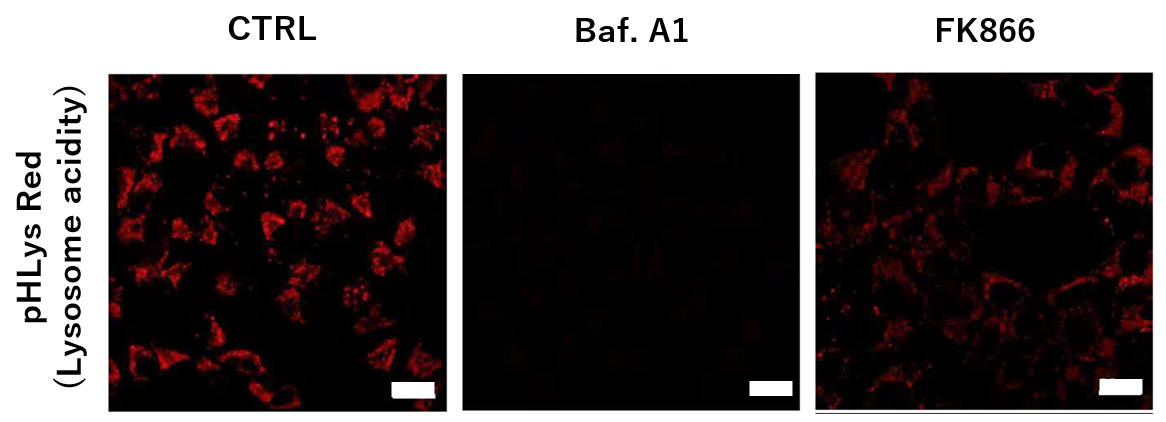
-
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Wash x2 (HBSS)
↓
. pHLys Red working solution (HBSS, 1,000 times dilution)
. pHLys Red working solution (HBSS, 1,000 times dilution) + 50 nM Bafilomycin
A1
. pHLys Red working solution (HBSS, 1,000 times dilution) + 10 nM FK866
↓
30 min, 37°C
↓
Observed by confocal microscopy (x20)
|
Simultaneous Detection of Lysosomal and Mitochondrial Dysfunction
-
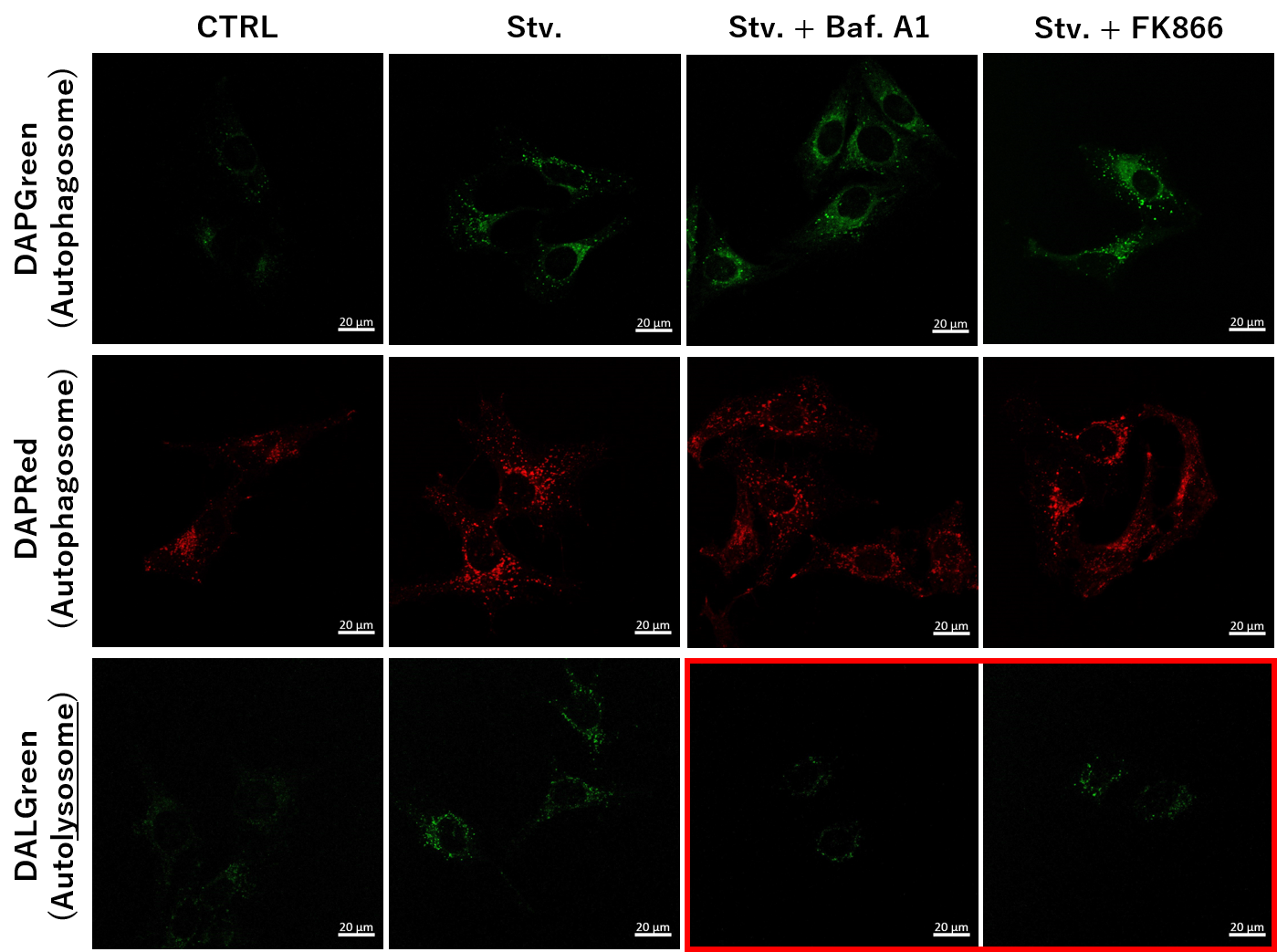
-
We tried the simultaneous detection of lysosomal and mitochondrial dysfunction in Hela cells treated with CCCP or Antimycin (AN). CCCP and AN are well-known inducers of mitochondrial ROS regarding loss of mitochondrial membrane potential. Recent research showed the result that CCCP induces not only mitochondrial ROS but also lysosomal neutralization. To detect mitochondrial ROS, HeLa cells were labeled by MitoBright ROS Deep Red - Mitochondrial Superoxide Detection, and the lysosomal mass and pH were detected separately with LysoPrime Green and pHLys Red. Co-staining with MitoBright ROS and Lysosomal dyes demonstrated that CCCP causes lysosomal neutralization and mitochondrial ROS induction at the same time.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Single-stain with 2 umol/I DALGreen or
0.2 umol/I DAPGreen or
0.2 umol/I DAPRed
30 min, 37℃
↓
Wash x2 (MEM, FBS+)
↓
. Control (MEM, FBS+) for 2 h 20 min
· Starvation (HBSS) for 2 h 20 min
· Stv.2 h = 10 nM Bafilomycin A1 (HBSS) for 20 min
· Stv.2 h = 10 nM FK866 (HBSS) for 20 min
Observed by confocal microscopy (x40)
|

















