Ferroptosis: Mechanisms in Disease and Kit Selection
Topics
- What is Ferroptosis?
- How Does Ferroptosis Cause Cell Death?
- Research on Related Diseases
- Ferroptosis-Related Reagent Selection Guide
- Experimental Example: Evaluating intracellular uptake and redox balance in erastin-induced ferroptosis
- Experimental Example: Changes in various indicators of cell death induced by drugs
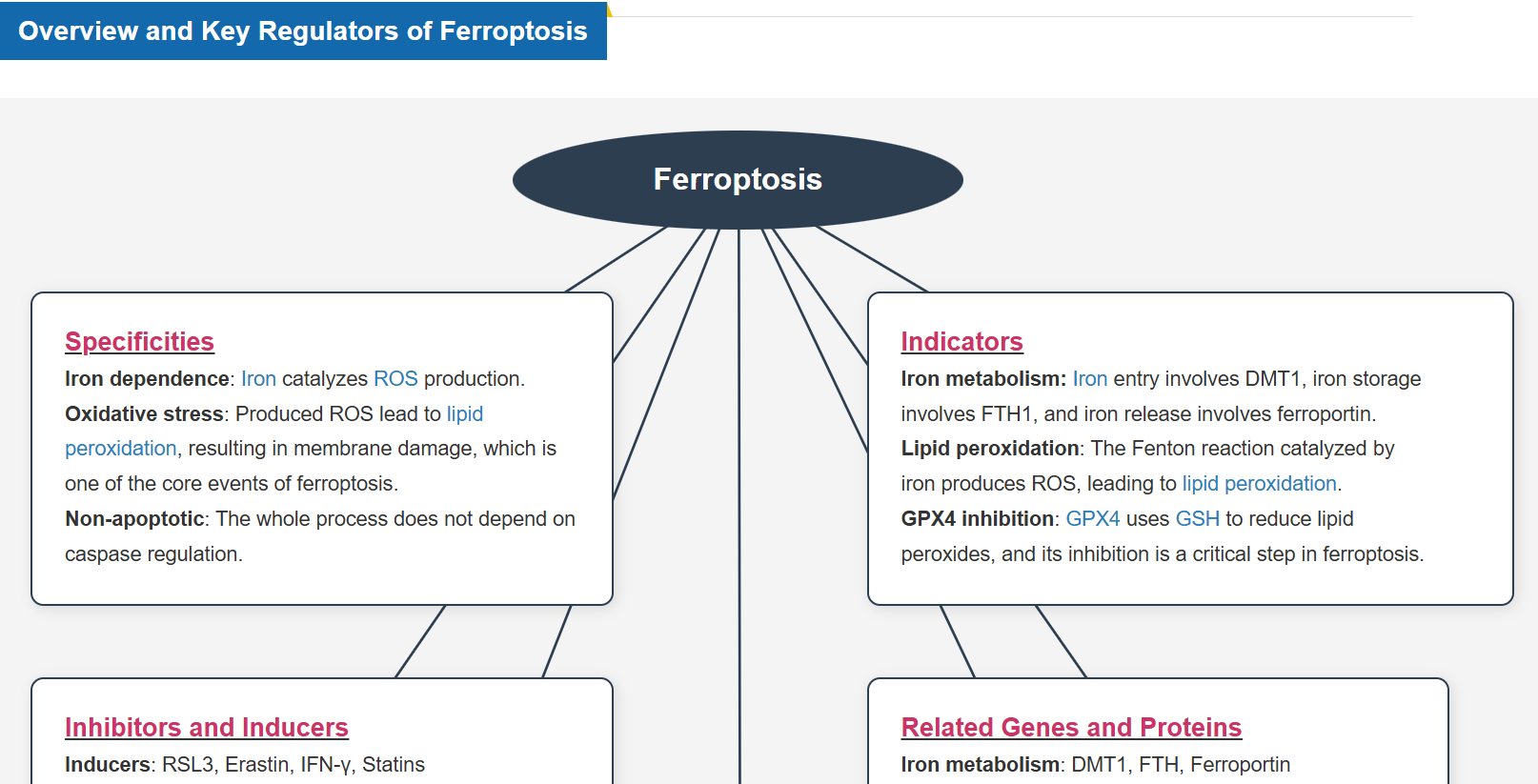
What is Ferroptosis?
“Ferroptosis” was coined by Stockwell et al. at Columbia University in 2012 and described as a form of iron-dependent cell death. * It was reported to be a form of programmed cell death by the Nomenclature Committee on Cell Death (NCCD) in 2018.
Ferroptosis is a form of programmed cell death caused by iron ion-dependent accumulation of lipid peroxides. Ferroptosis has been shown to follow a different cell death pathway from apoptosis and thus is attracting attention as a new target for cancer therapy. It has also been found to be associated with various diseases, such as neurodegenerative diseases, cerebral apoplexy, and hepatitis (MASH).
*S. J. Dixon, B. R. Stockwell, et al., Ferroptosis: an iron-dependent form of nonapoptotic cell death., Cell, 2012, 149(5), 1060.
How Does Ferroptosis Cause Cell Death?
-
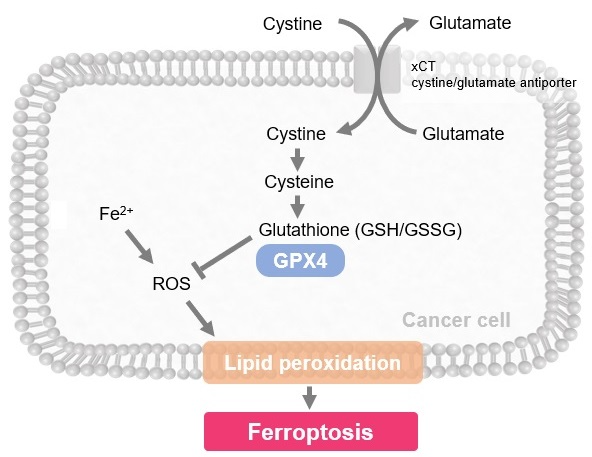
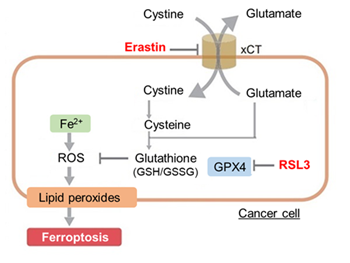
Ferroptosis is characterized by the accumulation of lipid peroxides. Lipid peroxides are formed from oxidation of polyunsaturated fatty acids (PUFA) in membrane phospholipids, with iron suggested to be involved. Intracellular glutathione peroxidase 4 (GPX4) uses reduced glutathione (GSH), an antioxidant, to reduce lipid peroxides generated by reactive oxygen species (ROS).*
However, when lipid peroxides accumulate due to GPX4 disruption or GSH depletion, ferroptosis is triggered.*Stockwell et al, a leading researcher in the field of ferroptosis, summarized inhibitors, inducers, and detection indicators of ferroptosis in the following review, in which Dojindo’s Liperfluo is introduced for detection of lipid peroxides.
B. R. Stockwell, et al., "Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease.", Cell, 2017, 171, 273.
Research on Related Diseases
-
Metabolic dysfunction-associated steatohepatitis (MASH) Suppression of hepatitis via ferroptosis
In a study involving the livers of MASH model mice, it was confirmed that necrosis precedes apoptosis in the development of fatty liver. Further experiments showed that ferroptosis is involved within necrosis as a trigger for steatohepatitis and that inhibition of ferroptosis almost completely suppressed the onset of hepatitis.
Minoru Tanaka, et al., "Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis", Cell Death & Disease, 2019, 10, 449.
Related article: changes in intracellular markers associated with MASH
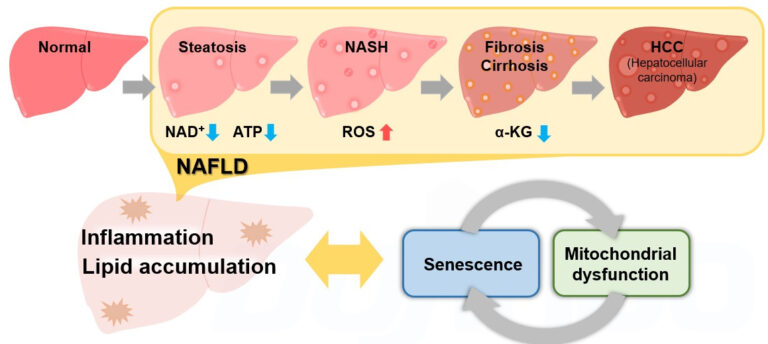
The article summarizes reports on changes in each indicator of metabolic states and cellular senescence using the NASH model.
(Click on the “MASH” tab in the link)
Experimental example: measurement of intracellular metabolism in MASH model tissue
Measurement of ATP, a-KG, and NAD levels in liver tissue of high-fat diet-treated type 1 diabetic model mice. (Please refer to each product’s website for more information, “Experimental Example: Change in Metabolism in Liver Tissue of MASH-Induced Mouse”)
-
Neurodegenerative disease Confirmation of the link between lysosomal disorders and ferroptosis
In experiments using human neurons, it is reported that knockdown of the lysosomal protein prosaposin induces formation of lipofuscin, a hallmark of aging. This process involves the iron-catalyzed generation of reactive oxygen species, leading to induction of ferroptosis.
Martin Kampmann, et al., "Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis", Nature Neuroscience, 2021, 24, 1020
Cancer Regulation of cancer immunity via ferroptosis
CD8+ T cells activated by immunotherapy were found to confer an anti-tumor effect by promoting lipid peroxidation and inducing ferroptosis. The mechanism of immunotherapy-induced inhibition of cystine uptake and promotion of lipid peroxidation in tumor cells is discussed.
Weiping Zou, et al, "CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy", Nature, 2019, 569, 270
Ferroptosis-Related Reagent Selection Guide
Lipid Peroxide and Iron (Fe2+) Detection Reagents
| Name | Liperfluo | MitoPeDPP | MDA Assay Kit | Mito-FerroGreen | FerroOrange | |
|---|---|---|---|---|---|---|
| Target | Lipid Peroxide | Lipid Peroxidation | Lipid Peroxidation | Malondialdehyde | Ferrous Ion(Fe2+) | Ferrous Ion(Fe2+) |
| Localization | Intracellular | Mitochondria | Intracellular | Intracellular | Mitochondria | Intracellular |
| Detection (Fluorescence:Ex/Em) |
Fluorescence (524 nm/535 nm) |
Fluorescence (452 nm/470 nm) |
Fluorescence 1. 488 nm/510-550nm 2. 561 nm/600-630nm |
Fluorescence (540 nm/590 nm) Colorimetric: 532 nm |
Fluorescence (505 nm/580 nm) |
Fluorescence (543 nm/580 nm) |
| Instrument | Fluorescence Microscope, FCM |
Fluorescence Microscope, FCM |
Fluorescence Microscope, FCM, Microplate Reader |
Microplate Reader | Fluorescence Microscope, Microplate Reader |
Fluorescence Microscope |
| Sample | Live Cell | Live Cell | Live Cell | Cell, Tissue | Live Cell | Live Cell |
Oxidative Stress- and Metabolism-Related Reagents and Kits
| Name | ROS Assay Kit -Highly Sensitive DCFH-DA- |
GSSG/GSH Quantification Kit | Glutamine Assay Kit-WST | Glutamate Assay Kit-WST |
|---|---|---|---|---|
| Target | ROS (Reactive oxygen species) | Glutathione (oxidized/reduced) | Glutamine | Glutamine |
| Localization | Intracellular | Intracellular | Intracellular/Extracellular | Intracellular/Extracellular |
| Detection (Fluorescence:Ex/Em) |
Fluorescence (505 nm/525 nm) |
Colorimetric:412 nm | Colorimetric:450 nm | Colorimetric:450 nm |
| Instrument | Fluorescence Microscope, FCM, Microplate Reader |
Microplate Reader | Microplate Reader | Microplate Reader |
| Sample | Live Cell | Cell, Tissue, Blood Plasma, Red Blood Cell | Cell, Culture Medium | Cell, Culture Medium |
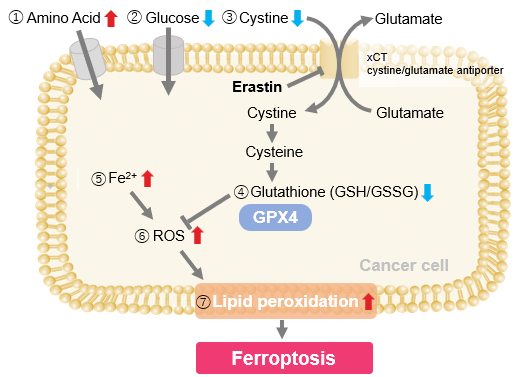
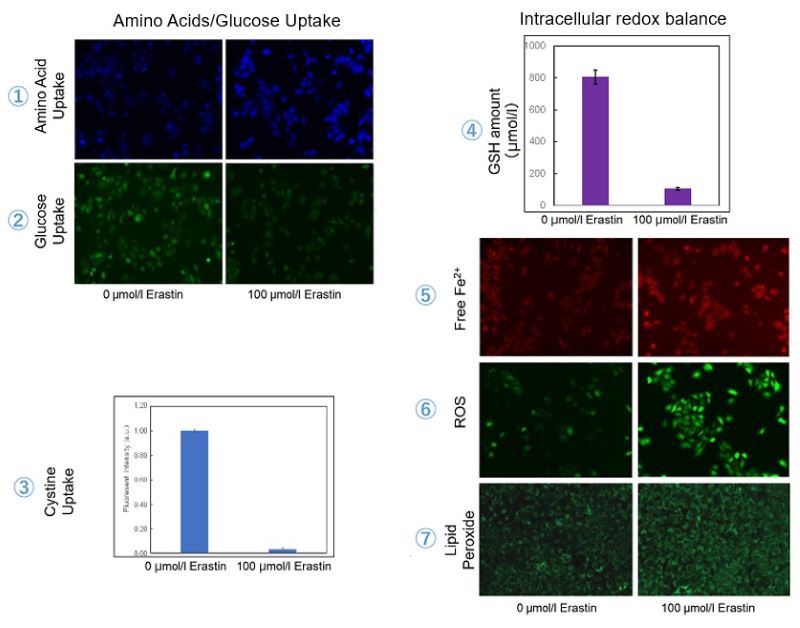
Experimental Example: Evaluating intracellular uptake and redox balance in erastin-induced ferroptosis
|
|
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following. Results Cell Line: A549 |
|
|
Products in Use |
||
Experimental example: Changes in various indicators of cell death induced by drugs
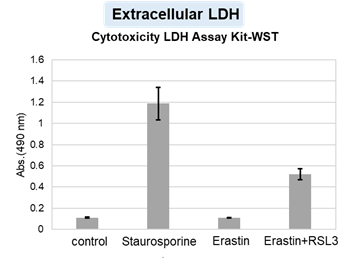
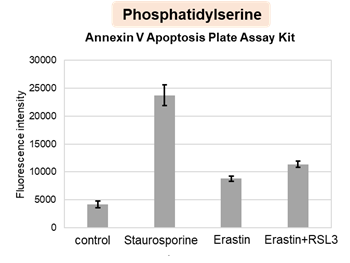
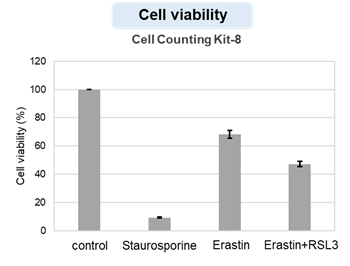
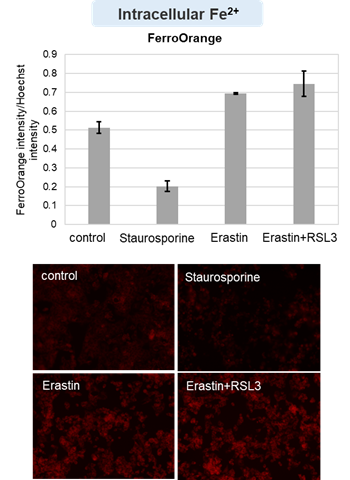
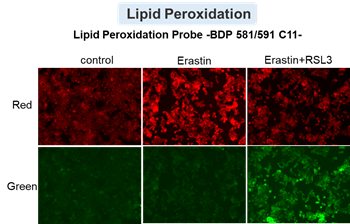
HepG2 cells treated with the apoptosis-inducing agent staurosporine or the ferroptosis-inducing agents Erastin and RSL3. After treatment, extracellular LDH, phosphatidylserine, cell viability, intracellular Fe2+ and lipid peroxidation were determined.
The results showed that apoptosis-induced cells treated with staurosporine showed an increase in phosphatidylserine, a decrease in cell viability and an increase in extracellular LDH, indicating that cell death had occurred. On the other hand, intracellular Fe2+, an indicator of ferroptosis, remained unchanged. In cells treated with Erastin, a ferroptosis inducer, intracellular Fe2+ increased and cell viability decreased, but extracellular LDH and lipid peroxidation (lipid peroxidation: decrease in red fluorescence and increase in green fluorescence) did not increase. In cells in which ferroptosis was more strongly induced by co-treatment with RSL3 in addition to Erastin, increased intracellular Fe2+ and lipid peroxidation were observed. Moreover, decreased cell viability and increased dead cells were detected. Meanwhile, phosphatidylserine showed a lower rate of increase during ferroptosis induction compared to apoptosis-induced cells. These results suggest that cell death can be distinguished by evaluating a combination of cell death indicators.
[Products in use]
Extracellular LDH : Cytotoxicity LDH Assay Kit-WST (Product code: CK12)
Phosphatidylserine: Annexin V Apoptosis Plate Assay Kit(Product code: AD12)
Cell viability : Cell Counting Kit-8 (Product code: CK04)
Intracellular Fe2+ : FerroOrange (Product cose: F374) *Normalized with Hoechst 33342 fluorescence intensity
Lipid peroxidation : Lipid Peroxidation Probe -BDP 581/591 C11- (Product code: L267)
[Experimental conditions]
Cell type: HepG2 cell(2×104 cells/well)
Drugs: Staurosporin(5 μmol/l), Erastin(25 µmol/l), Erastin+RSL3(both 25 µmol/l) *Diluted in serum-free medium