Lysosomes Keep Mitochondria Healthy [Mar. 18, 2025]
Previous Science Note
|
Lysosomes play a critical role in supporting mitochondria and maintaining mitochondrial homeostasis. This Science Note introduces the latest findings on the intimate relationship between lysosomes and mitochondria, including the mechanism by which lysosomes partially remove mitochondrial components, the process of mRNA transport in neuronal axons, and the compensatory mechanisms that occur in response to lysosomal dysfunction. |
||||||||||||||||||||
|
Lysosomes drive the piecemeal removal of mitochondrial inner membrane (Nature, 2024)
Lysosomes maintain mitochondrial quality control not only by degrading the entire mitochondria but also by selectively removing damaged sections of the inner mitochondrial membrane (IMM). The damaged IMM protrudes through pores in the outer membrane formed by VDAC1, and nearby lysosomes engulf this IMM portion, leading to the formation of vesicles in the cytoplasm. Highlighted technique: This study uses a combination of super-resolution microscopy, live-cell imaging, and specific fluorescent probes and proteins to visualize and track in real time the dynamic process of IMM protruding through the OMM and their subsequent uptake by lysosomes. Related technique Lysosomal function, Mitophagy detection |
||||||||||||||||||||
|
Neurons have long axons and require local protein synthesis, which relies on lysosome-related vesicles to transport mRNA within the axon. Disruption of this transport leads to mitochondrial dysfunction and axonal degeneration, suggesting that abnormalities in mRNA transport by lysosome-related vesicles may trigger neurodegenerative diseases. Highlighted technique: In in vitro experiments with neuronal cells, it is critical to accurately mimic real neurons. While neuronal cell lines often differ in morphology from neurons in vivo, this study uses iPS-derived or primary neurons and employs a microfluidic device to more accurately replicate axons and assess intracellular dynamics. Related technique Mitochondrial staining, Mitochondrial superoxide detection |
||||||||||||||||||||
|
Dysfunctional mitochondria are normally removed by lysosomes, but when lysosomal function is impaired, an alternative compensatory mechanism facilitates the secretion of accumulated mitochondria into extracellular vesicles (EVs). These EVs containing mitochondria are taken up by macrophages and degraded without triggering inflammation. Highlighted technique: CD81 and CD63 are markers for extracellular vesicles (EVs) and are key molecules widely used for tracking, quantifying, identifying, and purifying EVs within cells. In addition, membrane staining dyes are often used in experiments to track the uptake of purified EVs by cells or organisms. Related technique Oxygen consumption rate assay, Exosome staining |
||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||
|
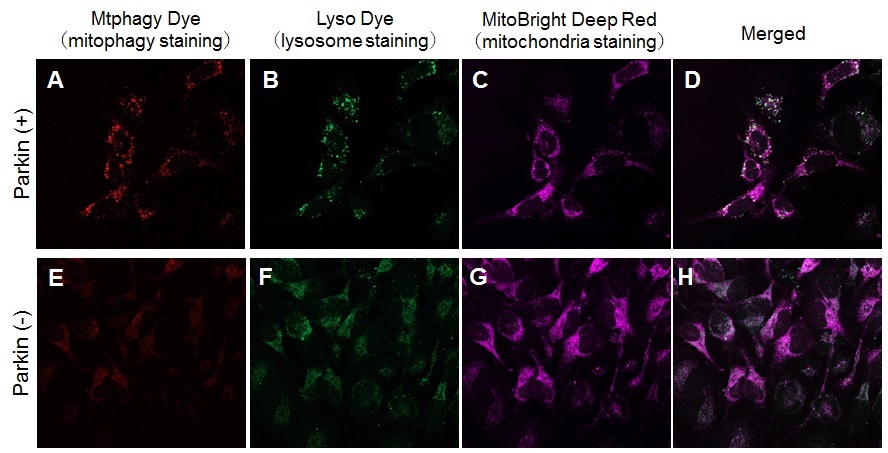
Induction of mitophagy by carbonyl cyanide m-chlorophenyl hydrazone (CCCP) as a mitochondrial-uncoupling reagent with Parkin expressed HeLa cells HeLa cells were seeded on μ-slide 8 well (Ibidi) and cultured at 37oC overnight in a 5%-CO2 incubator. The cells were transfected with Parkin plasmid vector by HilyMax transfection reagent, and incubated at 37oC overnight. The Parkin expressed HeLa cells were washed with Hanks’ HEPES buffer twice and then incubated at 37oC for 30 minutes with 250 μl of 100 nmol/l Mtphagy Dye working solution containing 100 nmol/l MitoBright LT Deep Red. After the washing of the cells with Hanks’ HEPES buffer twice, the culture medium containing 10 μmol/l CCCP was added to the well. After 24 hours incubation, mitophagy was observed by a fluorescence microscopy. After removing the supernatant, 250 μl of 1 μmol/l Lyso Dye working solution were added to the cells and incubated at 37oC for 30 minutes. The cells were washed with Hanks’ HEPES buffer twice and then co-localization of Mtphagy, Lyso Dye and MitoBright Deep Red was observed by confocal fluorescence microscopy. |
|||
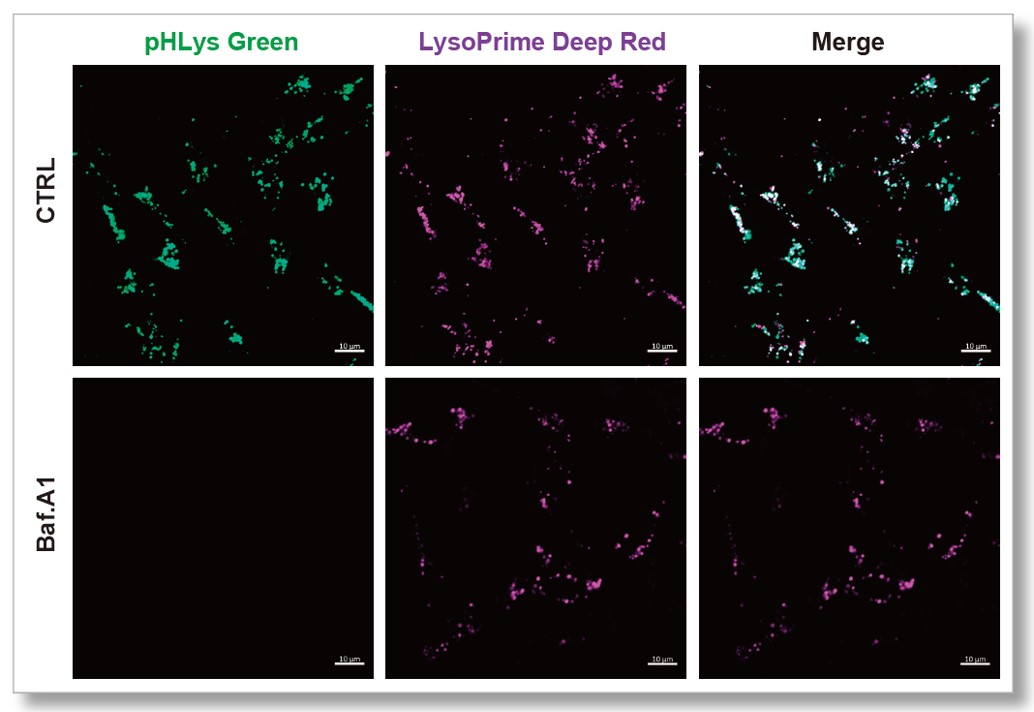
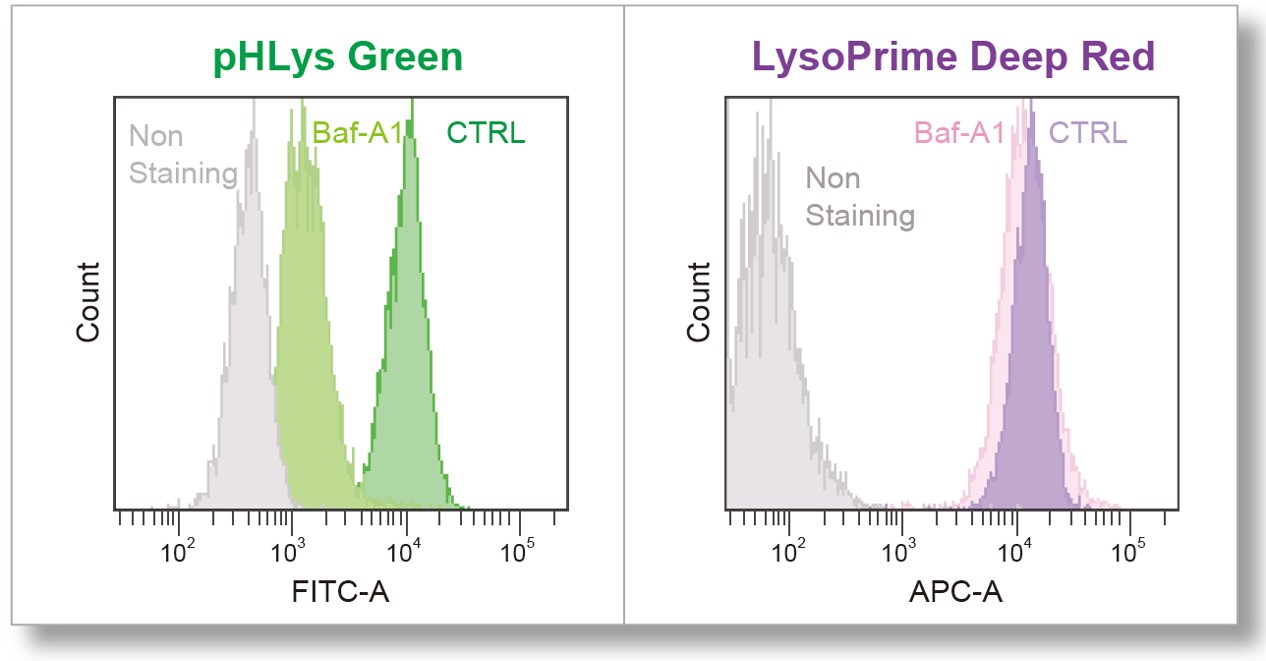
| > Effect of Bafilomycin A1 on Lysosomal Mass and pH HeLa cells were used to detect changes in lysosomal mass and pH when treated with the lysosomal acidification inhibitor Bafilomycin A1 (Baf. A1). The fluorescence of LysoPrime Deep Red has no change regardless of the addition of Baf. A1, while the fluorescence of pHLys Green decreased due to lysosomal neutralization caused by the addition of Baf. A1. This indicates that Baf. A1 does not affect lysosomal mass, although lysosomal function is reduced. |
|||
 pHLys Green (Green) : Ex=488 nm, Em=490-550 nm LysoPrime Deep Red (Violet) : Ex=633 nm, Em=640-700 nm |
 pHLys Green (FITC Filter) : Ex=488 nm, Em=515-545 nm LysoPrime Deep Red |
||