|
Scientists have discovered that disablement of fatty acid oxidation in cardiomyocytes improves resistance to hypoxia and stimulates cardiomyocyte proliferation, allowing heart regeneration after ischaemia–reperfusion injury. This process involves epigenetic inhibition of cardiomyocyte maturation via activation of the alpha-ketoglutarate-dependent lysine demethylase KDM5. |
|
Inhibition of fatty acid oxidation enables heart regeneration in adult mice Point of Interest - Cardiomyocytes undergo postnatal maturation which limits heart regeneration. - Disabling fatty acid oxidation in these cells promotes their proliferation and aids heart repair after injury. - This process is linked to changes in energy metabolism and the activation of KDM5, altering gene expression. - Reversing metabolic maturation can rejuvenate cardiomyocytes and facilitate heart healing. |
| Related Techniques |
|
|
|
|
|
|
| Related Applications |
<Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism > |
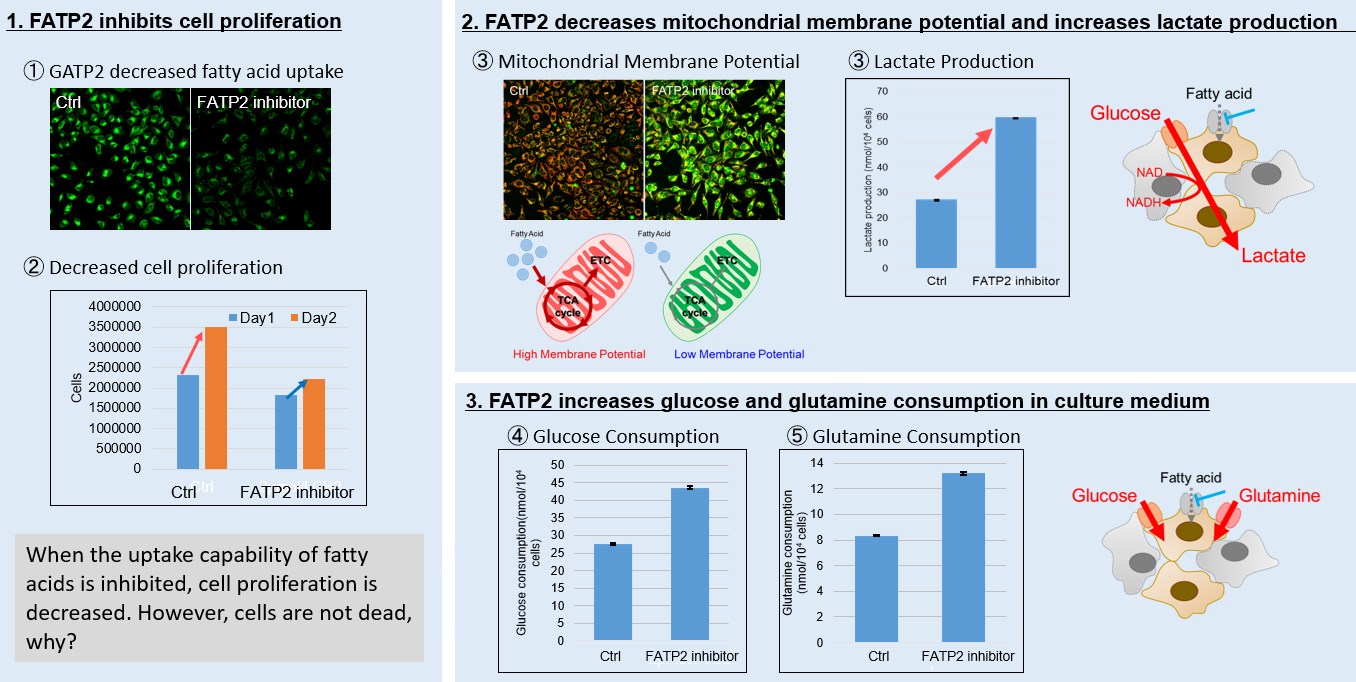
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells:・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1). ・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2) ・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3)
|