|
Metabolic alterations play a critical role in the development and progression of diseases such as cancer, neurodegeneration and senescence. In cancer, altered metabolism supports rapid cell growth and survival, often through the Warburg effect, where cancer cells rely heavily on glycolysis even in the presence of oxygen. Neurodegenerative diseases such as Alzheimer's and Parkinson's are associated with dysregulated energy metabolism leading to impaired neuronal function and increased oxidative stress. Senescence, the process of cellular aging, involves metabolic reprogramming that contributes to the cessation of cell division and the secretion of pro-inflammatory factors, affecting tissue homeostasis and promoting age-related diseases.
|
-
Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling
Click here for the original article: Zhengnan Cai, et. al., Cellular & Molecular Immunology, 2024.
Point of Interest
- IL-4 and tumor media upregulate phosphoglycerate dehydrogenase (PHGDH), promoting immunosuppressive M2 macrophage activation and proliferation.
- PHGDH-mediated serine biosynthesis activates mTORC1 signaling and maintains M2-like macrophages.
- Loss of PHGDH disrupts metabolism and shifts M2-like tumor-associated macrophages to an M1-like phenotype, suggesting that PHGDH inhibition may suppress tumor progression.
-
LXR/CD38 activation drives cholesterol-induced macrophage senescence and neurodegeneration via NAD+ depletion
Click here for the original article: Ryo Terao, et. al., Cell Reports, 2024.
Point of Interest
- Metabolic and genotoxic stress promotes lysosomal cholesterol efflux and NAD+ depletion in macrophages via LXR/CD38 signaling.
- Cholesterol-mediated NAD+ depletion induces macrophage senescence, contributing to age-related macular degeneration (AMD) and neurodegeneration.
- NAD+ augmentation and senolytics reverse macrophage senescence, preventing AMD and neurodegeneration.
-
TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer’s disease mouse models
Click here for the original article: Edward N. Wilson, et. al., Nat Neurosci., 2024.
Point of Interest
- Defective myeloid responses in late-onset AD are associated with aging and triggering receptor expressed on myeloid cells 1 (TREM1), a pro-inflammatory factor, influences cognitive decline.
- The age-related increase in TREM1 suppresses glucose metabolism, particularly the production of ribose-5P, which is essential for both glycolysis and the biosynthesis of purines, pyrimidines and NAD+, leading to disruption of homeostatic microglial metabolism and immune function.
- TREM1 deficiency prevents age-related changes in myeloid metabolism, inflammation and hippocampal memory function.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
Comparison of metabolic pathway in two types of cancer cells
The dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values.
Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines.
|
<Evaluation by Lactate production and ATP levels>
-
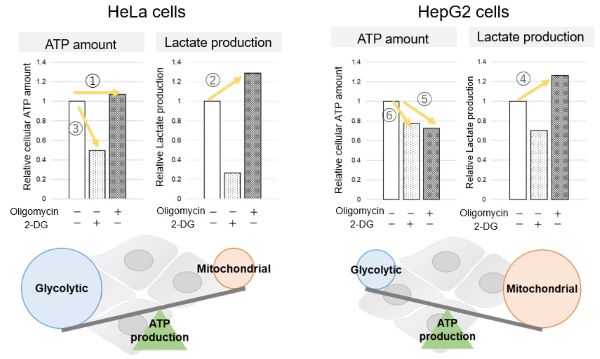
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
-
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
-
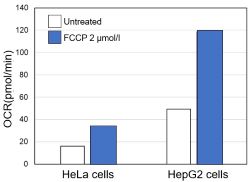
<Evaluation by OCR value>
-
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|


















