|
Targeting metabolic heterogeneity in the tumor microenvironment (TME) is critical in cancer research because metabolic diversity affects cancer and immune cell function, promoting tumor progression and immune suppression. In addition to previous findings, recent studies have identified acetyl-CoA carboxylase and glycosphingolipid synthesis as key mediators of immune evasion in the TME. In this note, we introduce a review of cancer-promoting mechanisms driven by metabolic heterogeneity in the TME, along with recent findings.
|
-
Metabolic programming and immune suppression in the tumor microenvironment
Click here for the original article: Emily N. Arner, et. al., Cancer Cell, 2023.
Point of Interest
- Metabolic heterogeneity in the tumor microenvironment (TME) arises from cellular diversity and affects the metabolic programs of cancer and immune cells, promoting tumor growth, metastasis, and immune evasion.
- Altered nutrients and signals in the TME suppress effector immune cells and favor regulatory immune cells, contributing to immune suppression.
- Targeting metabolic heterogeneity in the TME is a promising strategy to overcome immune suppression and improve the efficacy of cancer immunotherapy.
-
Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment
Click here for the original article: Elizabeth G. Hunt, et. al., Cell Metabolism, 2024.
Point of Interest
- T cells in the solid tumor microenvironment (TME) must catabolize lipids via mitochondrial fatty acid oxidation (FAO) to provide energy during nutrient stress.
- The solid TME activates acetyl-coenzyme A carboxylase (ACC) in T cells, causing lipid droplet accumulation in tumor-infiltrating T cells, which inhibits FAO.
- Restricting ACC activity rewires T cell metabolism to maintain energy during TME stress.
-
Glycosphingolipid synthesis mediates immune evasion in KRAS-driven cancer
Click here for the original article: Mariluz Soula, et. al., Nature, 2024.
Point of Interest
- De novo sphingolipid synthesis is critical for immune evasion in KRAS-driven cancers.
- Blocking glycosphingolipid synthesis increases IFNGR1 levels and enhances IFNγ-induced growth arrest and pro-inflammatory signaling.
- Inhibiting glycosphingolipid production increases natural killer and CD8+ T cell activity and synergizes with checkpoint blockade therapy to enhance anti-tumor responses.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
Comparison of metabolic pathway in two types of cancer cells
The dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values.
Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines.
|
<Evaluation by Lactate production and ATP levels>
-
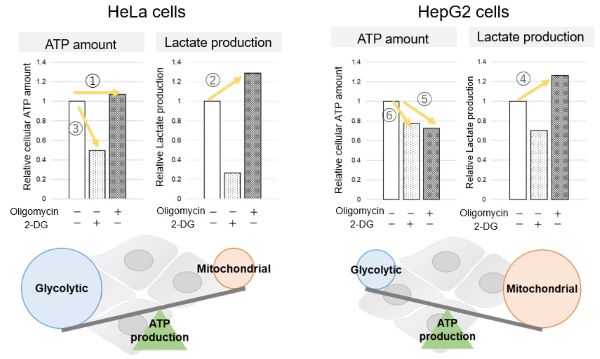
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
-
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
-
<Evaluation by OCR value>
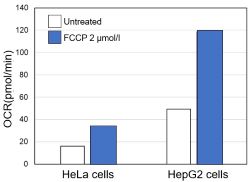 
-
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|


















