Liperfluo

Lipid Peroxide Detection
- Selective measurement of Lipid Peroxide
- Less cellular photo-damager
- Applicable for microscopy and FCM analysis
-
Product codeL248 Liperfluo
-
CAS No.1448846-35-2
-
Chemical nameN-(4-Diphenylphosphinophenyl)-N'-(3,6,9,12-tetraoxatridecyl)perylene-3,4,9,10-tetracarboxydiimide
-
MWC51H41N2O8P=840.85
| Unit size | Price | Item Code |
|---|---|---|
| 50 μg x 5 | $401.00 | L248-10 |
Description
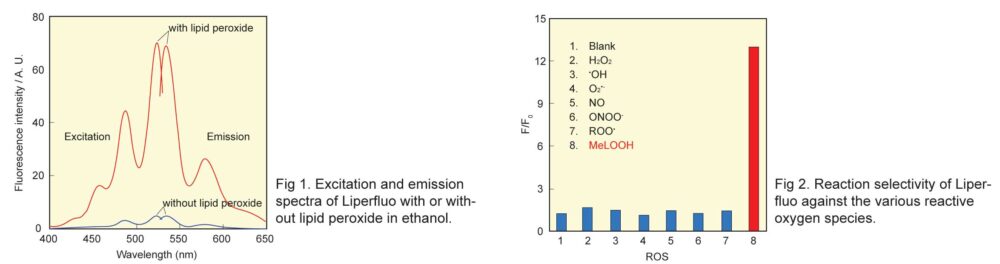
Liperfluo, a perylene derivative containing oligooxyethylene, is designed and exclusively developed by Dojindo for the detection of lipid peroxides. Liperfluo emits intense fluorescence by lipid peroxide specific oxidation in organic solvents such as ethanol. Among fluorescent probes that detect Reactive Oxygen Species(ROS), Liperfluo is the only compound that can specifically detect lipid peroxides. Since the excitation and emission wavelengths of the oxidized Liperfluo are 524 nm and 535 nm, respectively, photo-damage and auto-fluorescence from the sample can be minimized. The tetraethyleneglycol group linked to one end of diisoquinoline ring helps its solubility and dispersibility to aqueous buffer. Liperfluo’s oxidized form is nearly nonfluorescent in an aqueous media and emits a strong fluorescence in lipophilic sites such as in cell membranes. Therefore it can easily be applied to lipid peroxide imaging by a fluorescence microscopy and a flow cytometric analysis for living cells. Liperfluo is used to monitor lipid peroxidation in ferroptosis research.
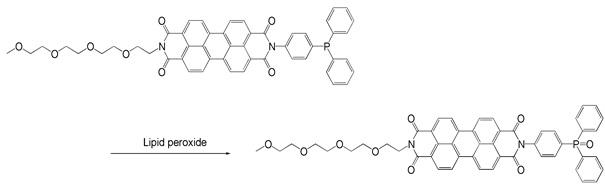
Reaction of Liperfluo with lipid peroxide
Ferroptosis Analysis Products
| Product Name | Target | Ditection Properties |
|---|---|---|
| FerroOrange | Intracellular Fe2+ | Microscopy, Plate reader Ex: 543 nm / Em: 580 nm |
| Mito-FerroGreen | Mitochondrial Fe2+ | Microscopy Ex: 505 nm / Em: 535 nm |
| Lyso-FerroRed | Lysosomal Fe2+ | Microscopy, FCM, Plate reader Ex: 551 nm / Em: 571 nm |
| Iron Assay Kit -Colorimetric- | Fe2+ and Fe3+ | Plate reader Colorimetric, λ: 593 nm |
| Liperfluo | Lipid Peroxide | Microscopy, FCM Ex: 488 nm / Em: 500-550 nm |
| Lipid Peroxidation Probe -BDP 581/591 C11- |
Lipid Peroxidation Process |
Microscopy, FCM, Plate reader |
| MDA Assay Kit | Malondialdehyde | Plate reader Fluorescence, Ex: 540 nm / Em: 590 nm Colorimetric, λ: 532 nm |
| Cystine Uptake Assay Kit | Cystine uptake | Plate reader Ex: 485 nm / Em: 535 nm |
| GSSG/GSH Quantification Kit | GSSG and GSH | Plate reader Colorimetric, λ: 405 nm |
Manual
Technical info
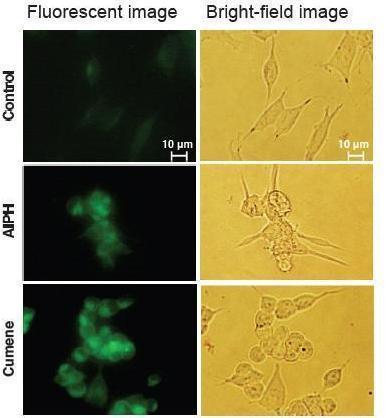
Live cell imaging of lipid peroxide
Procedure
1. Innoculate SH-SY5Y cells(6.0 x 105 cells/well) to a 6-well plate.
2. Incubate the plate at 37 ºC for overnight.
3. Add Liperfluo, DMSO solution (final conc. 20 μM) and incubate at
37 ºC for 15 min.
4. Add either Cumene Hydroperoxide (final conc. 100 μM) or AIPH*(final conc. 6 mM).
5. Incubate at 37 ºC for 2 hours.
6. Observe fluorescent by microscope**.
* AIPH: 2,2 Eazobis-[2-(2-imidazolin-2-yl)propane]dihydrochloride
** Olympus IX-71 epifluorescent microscope, mirror unit: U-MNIBA3, exposure time: 10 sec, ISO: 800
Data was kindly provided from Dr. N. Noguchi, Doshisha University, System Life Science Laboratory.
Cell line: L929
Microscope: Zeiss LSM510META
Filter type: FITC(GFP, Alexa488)wide filter
HFT UV/488
NFT490
BP505-550
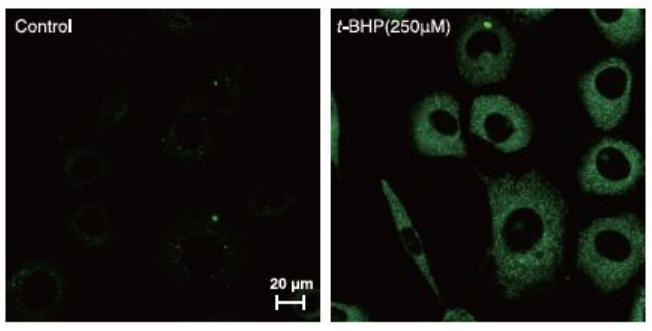
Procedure
1. Prepare cell suspension (2.5 x 105 cell/well) in 35mm Glass bottom dish and incubate at 37oC overnight in CO2.
2. Discard the media and add new media containing Liperfluo (final conc. 1μM) .
3. Incubate at 37oC for 30 min in CO2.
4. Discard the media add new media containing t-BHP (final conc. 250μM ).
5. Incubate at 37oC for 2 hours in CO2.
6. Observe using confocal microscope.
Data was kindly provided from Dr. T. Kumagai and Dr. H. Imai, Kitasato University, School of Pharmacy.
Flow cytometric analysis of lipid hydroperoxides in live cell
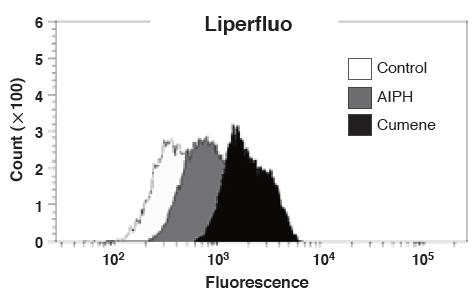
Procedure
1. Innoculate SH-SY5Y cells (6.0 x 105 cells/well) to a 6-well plate.
2. Incubate the plate at 37 ºC for overnight.
3. Add Liperfluo, DMSO solution (final conc. 20 μM) and incubate at 37 ºC for 15 min.
4. Add either Cumene Hydroperoxide (final conc. 100 μM) or AIPH*(final conc. 6 mM).
5. Incubate at 37 ºC for 2 hours.
6. Wash cells with PBS.
7. Collect cells with PBA and analyse by flow cytometer**.* AIPH: 2,2 Eazobis-[2-(2-imidazolin-2-yl)propane]dihydrochloride** BD FACSAriaTM I
Data was kindly provided from Dr. N. Noguchi, Doshisha University, System Life Science Laboratory.
Examples of use in ferotosis research
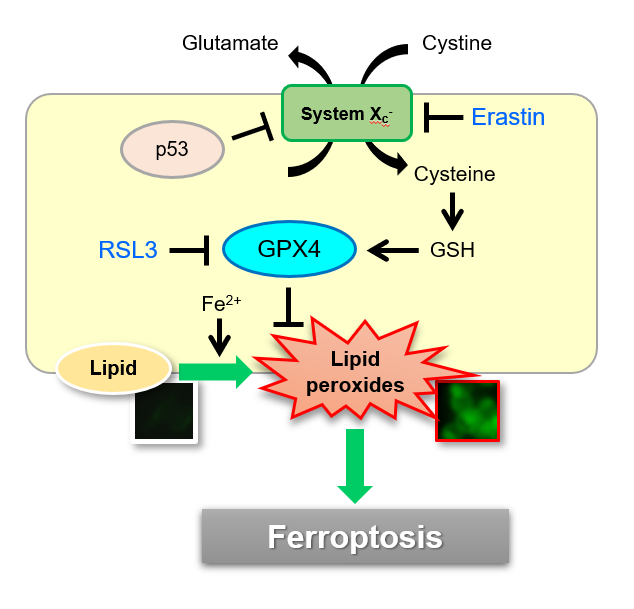
Necrosis, apoptosis and autophagy is known as cell death-related processes. In 2012, Ferroptosis was proposed as one of new cell deaths. Ferroptosis is studied as non apoptotic cell death caused by accumulation of iron ion-dependent lipid peroxide. Liperfluo is used as a fluorescent prove which can detect intracellular lipid peroxide directly.
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease.
B. R. Stockwell et al., Cell, 2017, 171(2), 273.
Oxidized Arachidonic/Adrenic Phosphatidylethanolamines Navigate Cells to Ferroptosis
V. E. Kagan et al., Nat. Chem. Biol., 2017, 13, (1), 81.
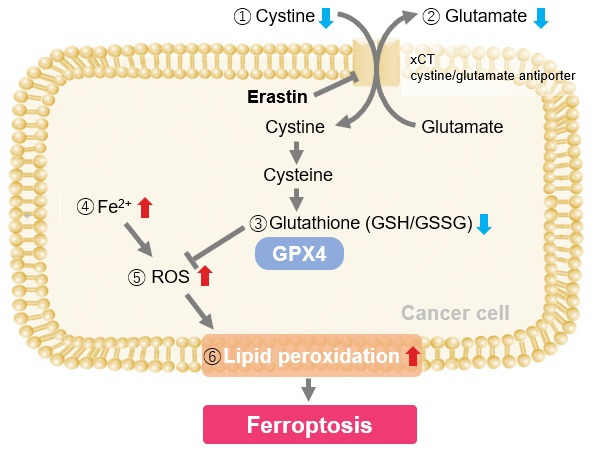
Induction of Ferroptosis by Erastin
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.

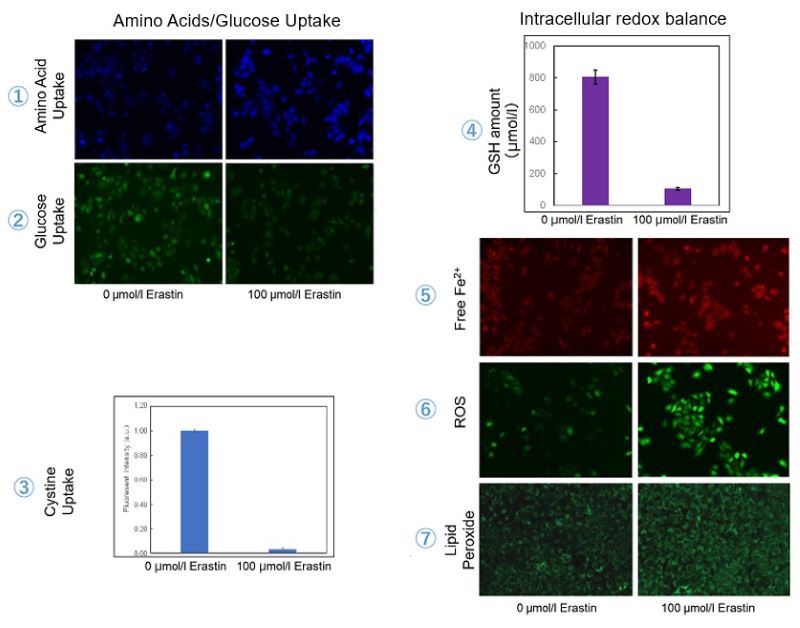
| 1 Amino Acid Uptake | : Amino Acid Uptake Assay Kit (Code: UP04) |
| 2 Glucose Uptake | : Glucose Uptake Assay Kit-Green (Code: UP02) |
| 3 Cystine Uptake | : Cystine Uptake Assay Kit (Code: UP05) |
| 4 Intracellular glutathione | : GSSG/GSH Quantification Kit (Code: G257) |
| 5 Intracellular labile Fe | : FerroOrange (Code: F374) |
| 6 Intracellular total ROS | : ROS Assay Kit -Highly Sensitive DCFH-DA- (Code: R252) |
| 7 Lipid Peroxides | : Liperfluo (Code: L248) |
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
References
1) N. Soh, T. Ariyoshi, T. Fukaminato, H. Nakajima, K. Nakano and T. Imato, "Swallow-tailed Perylene Derivative: a new Tool for Fluorescent Imaging of Lipid Hydroperoxides", Org. Biomol. Chem., 2007, 5, 3762.
2) K. Yamanaka, Y. Saito, J. Sakiyama, Y. Ohuchi, F. Oseto and N. Noguchi, "A Novel Fluorescent Probe with High Sensitivity and Selective Detection of Lipid Hydroperoxides in Cells", RSC Adv., 2012, 2, (20), 7894.
3) V. E. Kagan, G. W. Mao, F. Qu, J. P. F. Angeli, S. Doll, C. S. Croix, H. H. Dar, B. Liu, V. A. Tyurin, V. B. Ritov, A. A. Kapralov, A. A. Amoscato, J. Jiang, T. Anthonymuthu, D. Mohammadyani, Q. Yang, B. Proneth, J. K. Seetharaman, S. Watkins, I. Bahar, J. Greenberger, R. K. Mallampalli, B. R. Stockwell, Y. Y. Tyurina, M. Conrad and H. Bayır, "Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis", Nature Chemical Biology., 2017, 13, (1), 81.
4)Y. Nakashima, S. Ohta, A. M. Wolf, "Blue light-induced oxidative stress in live skin.", Free Radical Biology and Medicine., 2017, 108, 300.
5) M. Tsugita, N. Morimoto and M. Nakayama, "SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses", Particle and Fibre Toxicology., 2017, DOI 10.1186/s12989-017-0192-6.
6) K. Iuchi, A. Imoto, N. Kamimura, K. Nishimaki, H. Ichimiya, T. Yokota and S. Ohta, "Molecular hydrogen regulates gene expression by modifying the free radical chain reactiondependent generation of oxidized phospholipid mediators", Scientific Reports., 2016, DOI: 10.1038/srep18971.
7) A. J. Clark, and H. R. Petty., "WO3/Pt nanoparticles promote light-induced lipid peroxidation and lysosomal instability within tumor cells.", Nanotechnology., 2016, 27, (7), 075103.
8) T. Otani, M. Matsuda, A. Mizokami, N. Kitagawa, H. Takeuchi, E. Jimi, T. Inai and M. Hirata, "Osteocalcin triggers Fas/FasL-mediated necroptosis in adipocytes via activation of p300", Cell Death Dis., 2018, 9, 1194.
9) H.H. Dar, Y.Y. Tyurina, K. Mikulska-Ruminska, I. Shrivastava, H.C. Ting, V.A. Tyurin, J. Krieger, C.M. St Croix, S. Watkins, E. Bayir, G. Mao, C. Ambruster, A. Kapralov, H. Wang, M.H. Parsek, T.S. Anthonymuthu, A.F. Ogunsola, B.A. Flitter, C.J. Freedman, J.R. Gaston, T.R. Holman, J.M. Pilewski, J.S. Greenberger, R.K. Mallampalli, Y. Doi, J.S. Lee, I. Bahar, J.M. Bomberger, H. Bayır, V.E. Kagan, "Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium.", J. Clin. Invest.., 2018,DOI: 10.1172/JCI99490. .
10) H. Alborzinia, T. I. Ignashkova, F. R. Dejure, M. Gendarme, J. Theobald, S. Wolfl, R. K. Lindemann and J. H. Reiling , "Golgi stress mediates redox imbalance and ferroptosis in human cells", Commun Biol.., 2018, 1, (210), DOI: 10.1038/s42003-018-0212-6.
11) W. Wang, M. Green, J. E. Choi, M. Gijon, P. D. Kennedy, J. K. Johnson, P. Liao, X. Lang, I. Kryczek, A. Sell, H. Xia, J. Zhou, G. Li, J. Li, W. Li, S. Wei, L. Vatan, H. Zhang, W. Szeliga , W. Gu, R. Liu, T. S. Lawrence, C. Lamb, Y. Tanno, M. Cieslik, E. Stone, G. Georgiou, T. A. Chan, A. Chinnaiyan, W. Zou, "CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy.", Nature., 2019, 569(7755), 270-274.
12) M. Gao, J. Deng, F. Liu, A. Fan, Y. Wang, H. Wu, D. Ding, D. Kong, Z. Wang, D. Peer, Y. Zhao, 'Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy', Biomaterials., 2019, 233, 119486.
13) N. Wang, GZ. Zeng, JL. Yin, ZX. Bian, Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma', Biochem. Biophys. Res. Commun., 2019, 519, (3), 533-539.
14) T.Tsukui, Takayuki Tsukui, Z. Chen, H. Fuda, T. Furukawa, K. Oura, T. Sakurai, S. Hui, H. Chiba, 'Novel Fluorescence-Based Method To Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes', J. Agric. Food Chem., 2019, 67, (35), 9934-9941.
15) P. K. Mishra, A. Adameova, J. A. Hill, C. P. Baines, P. M. Kang, J. M. Downey, J. Narula, M. Takahashi, A. Abbate, H. C. Piristine, S. Kar, S. Su, J. K. Higa, N. K. Kawasaki and T. Matsui, Guidelines for evaluating myocardial cell death', Am. J. Physiol.: Heart Circ. Physiol. ., 2019, 317, (5), H891-H922 .
16) C. Liang, X. Zhang, M. Yang and X. Dong, Recent Progress in Ferroptosis Inducers for Cancer Therapy", Adv. Mater.., 2019,doi.org/10.1002/adma.201904197.
17) B. R. Stockwell and X. Jiang, The Chemistry and Biology of Ferroptosis", Cell Chem. Biol.., 2020, 27.
Q & A
-
Q
Which excitation filter or laser should I use for fluorescent microscope or flow cytometry?
-
A
Fluorescent microscope: GFP filter (ex. 450 – 490nm, em. 500 – 545nm)
FITC filter (ex. 467 – 498nm, em. 513 – 556nm)
Flow Cytometry: ex. 488nm
-
Q
Does phenol red or serum affect detection?
-
A
No, phenol red or serum will not affect detection. However, if there is high background, please use PBS instead.
-
Q
For high background or low fluorescence, is there anything I can do for improvement?
-
A
If the background is high, Liperfluo may be oxidized by light. Please avoid light during incubation by covering the solution with aluminum foil.
Increasing reaction time because of weak fluorescence will NOT improve the result due to increasing the background. Therefore, please adjust the device setting by following: increase the excitation light strength or exposure time.
-
Q
Can Liperfluo be used on both suspended and adherent cells? Fixed Cells?
-
A
Yes, Liperfluo can be used on both suspended and adherent cells.
We have data for HL-60 (suspended cells), CHO, and SH-SY5Y (adherent cells).
Liperfluo can NOT be used on fixed cells.
-
Q
Can I store Liperfluo (DMSO) solution?
-
A
No, Liperfluo in DMSO solution can NOT be stored due to instability of Liperfluo in light. After preparing the solution, please avoid light by using aluminum foil and use it within that day.
-
Q
What is recommended concentration of Liperfluo?
-
A
For cell staining, we recommend concentration between 1 to 10 μM and DMSO concentration lower than 1%.
Handling and storage condition
| Appearance: | Dark red crystalline powder or solid |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| NMR spectrum: | Authentic |
| 0-5°C, Protect from light, Nitrogen substitution |