ECGreen-Endocytosis Detection

Endocytosis Detection
- Precise visualization of endocytosis
- Track endocytosis using live cells
- High responsiveness to pH change
-
Product codeE296 ECGreen-Endocytosis Detection
| Unit size | Price | Item Code |
|---|---|---|
| 40 ul | $497.00 | E296-10 |
The general number of usable assays per 40 ul
– 35mm dish x 20
– μ-Slide 8 well x 20
Description
Endocytosis is a cellular process that involves macromolecules being taken up through a plasma membrane-derived vesicle called an endosome. The endocytic pathway contributes to the maintenance of intracellular homeostasis by bringing in various nutrients to the cell and transporting unwanted components to the lysosome, which acts as a waste disposal system. Recent findings reveal that disruption of endocytosis is related to certain neurodegenerative disorders and immune diseases. Consequently, investigation of the endocytic pathway is attracting considerable interest in the scientific community.
ECGreen-Endocytosis Detection is a pH dependent fluorescence dye that localizes to vesicle membrane. The visualization of endocytosis using the ECGreen is a more direct method than fluorescent analogs and allows visualization endocytosis from the stage of early endosomes.
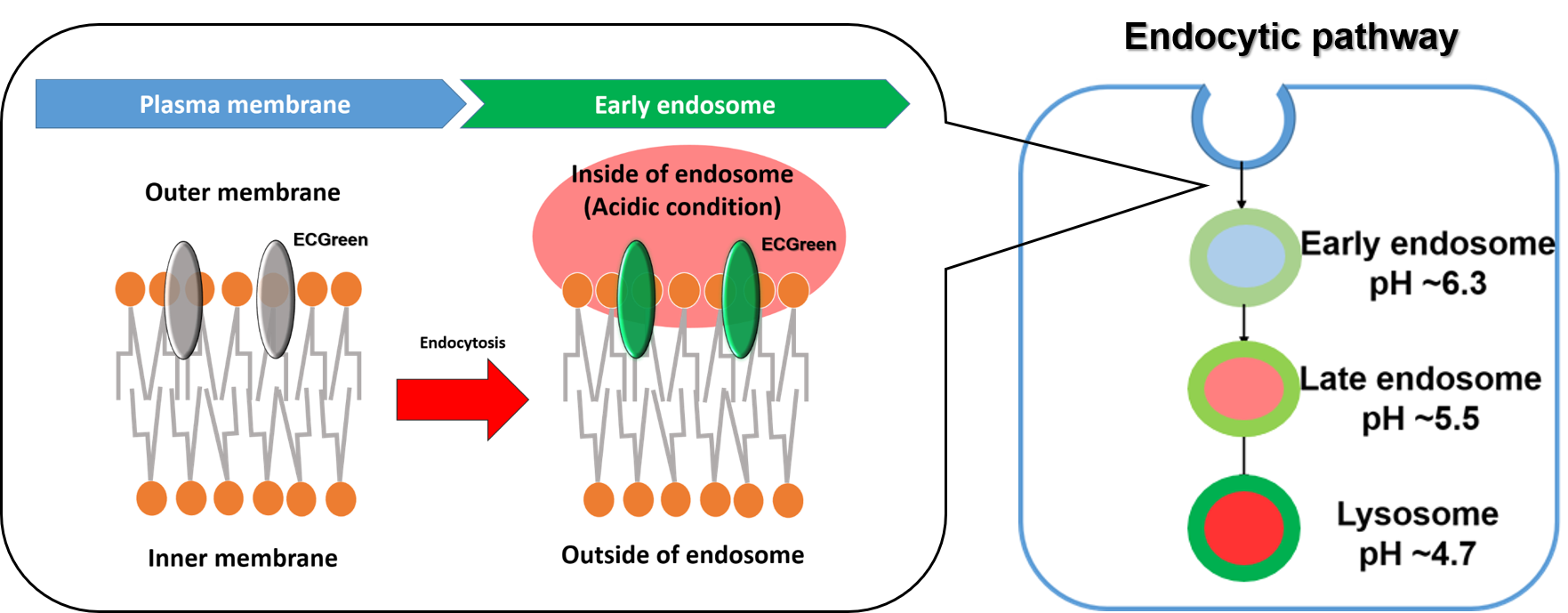
Figure 1. The detection mechanism of endocytosis
Manual
Technical info
Fluorescent Dye-Dextran Conjugates or membrane staining reagents are used to visualize endocytosis. However, they have limitations in observing the dynamics of endosomes in live cells in terms of precision of staining or retentivity of reagent. ECGreen is the reagent that over comes these limitations.
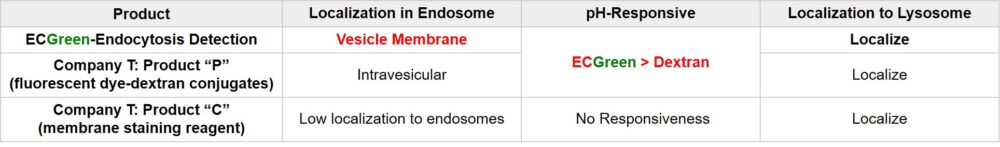
ECGreen-Endocytosis Detection is a pH dependent fluorescence dye that localizes to vesicle membrane. The visualization of endocytosis using the ECGreen is a more direct method than fluorescent analogs and allows visualization endocytosis from the stage of early endosomes.
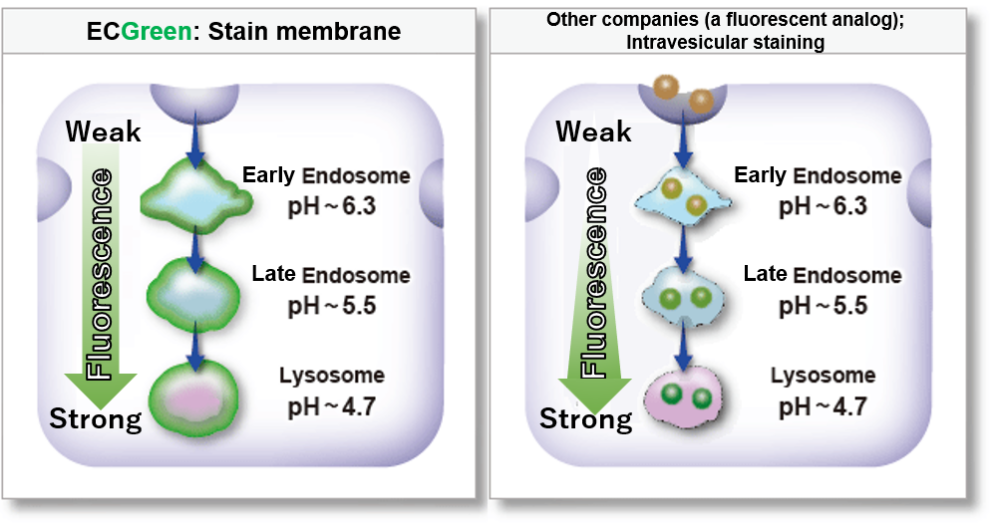
ECGreen-Endocytosis Detection is more sensitive to pH changes than exiting fluorescently labeled dextrans.
Therefore, ECGreen-Endocytosis Detection is appreciable for detection of early endosomes with high sensitivity.

Highly responsive to pH changes
ECGreen-Endocytosis Detection is more responsive to pH changes than fluorescently labeled dextran, which has been used in the past.
Therefore, even early endosomes can be detected with high sensitivity.
Change in fluorescence intensity at various pH
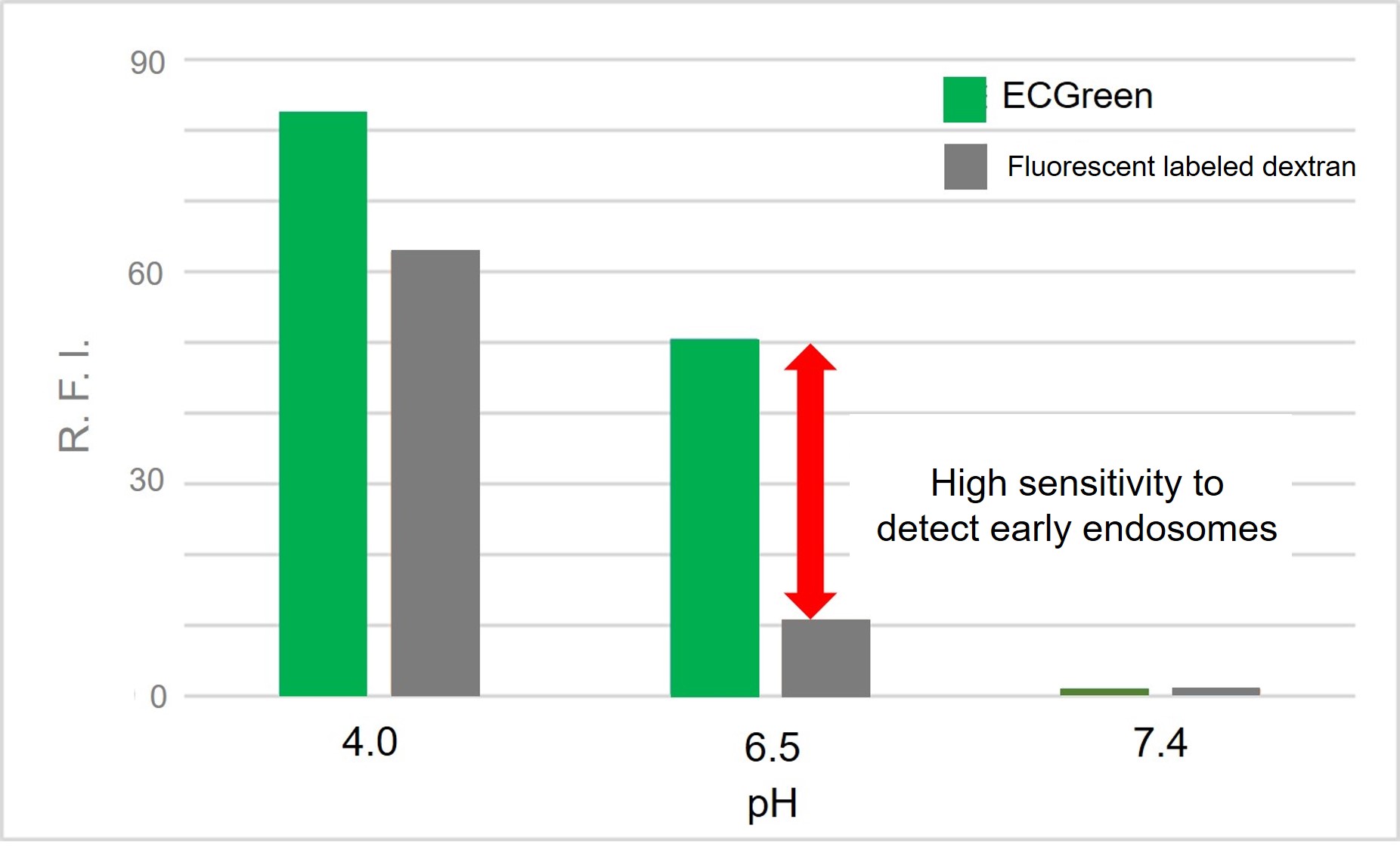
Fluorescence Property
λex: 386 nm, λem: 522 nm
<Observation conditions>
Ex : 350 ~ 410 nm, Em : 500 ~ 560 nm
Clear visualization of intracellular vesicular trafficking
Wortmannin is known to inhibit endosomal recycling and lysosomal translocation, leading to endosomal enlargement.
These changes induced by Wortmannin were confirmed by co-staining with ECGreen (green) and the following indicators.
①Eary endosome: Rab5-RFP (red)
② Recycling endosome: Fluorescent labeled Transferin (red)
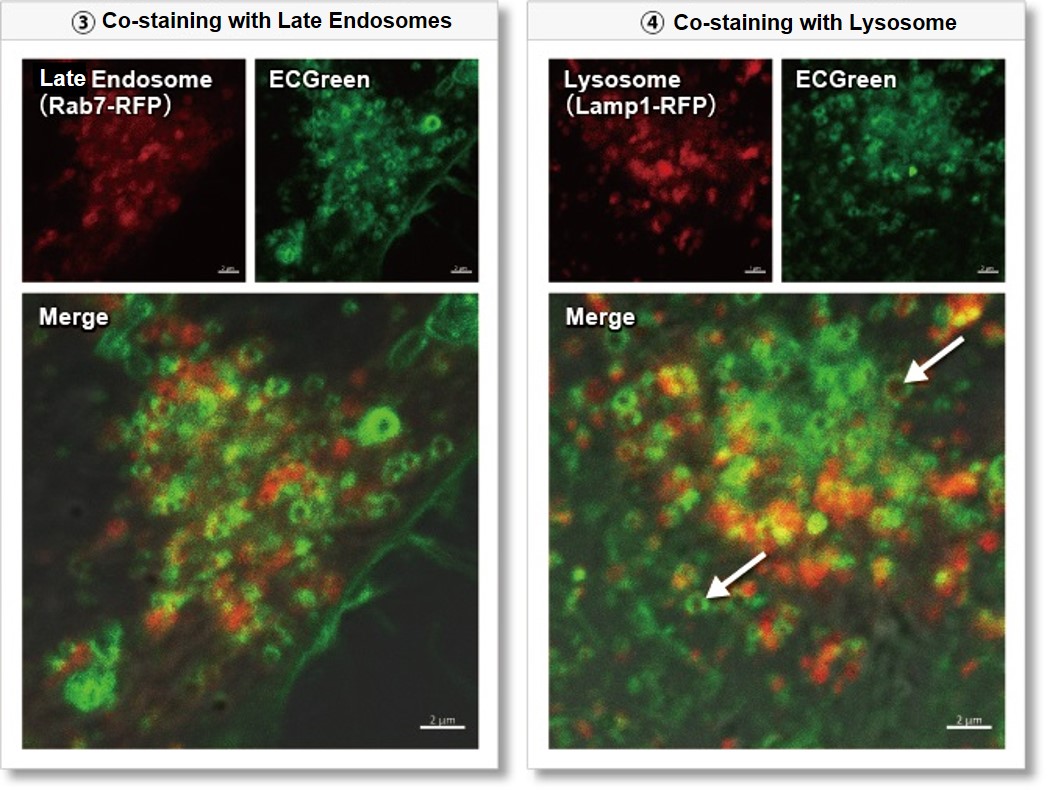
③ Late endosome: Rab5-RFP (red)
④ Lysosome: Lamp1-RFP (red)
As a result, it was confirmed that ECGreen (green) co-localizes only with enlarged early endosomes and recycling endosomes (Fig. ① and ②), but not with late endosomes or Lysosomes (Fig. ③ and ④), supporting Wortmannin's effect. ECGreen can visualize changes in the intracellular vesicular trafficking system and endosome shape.
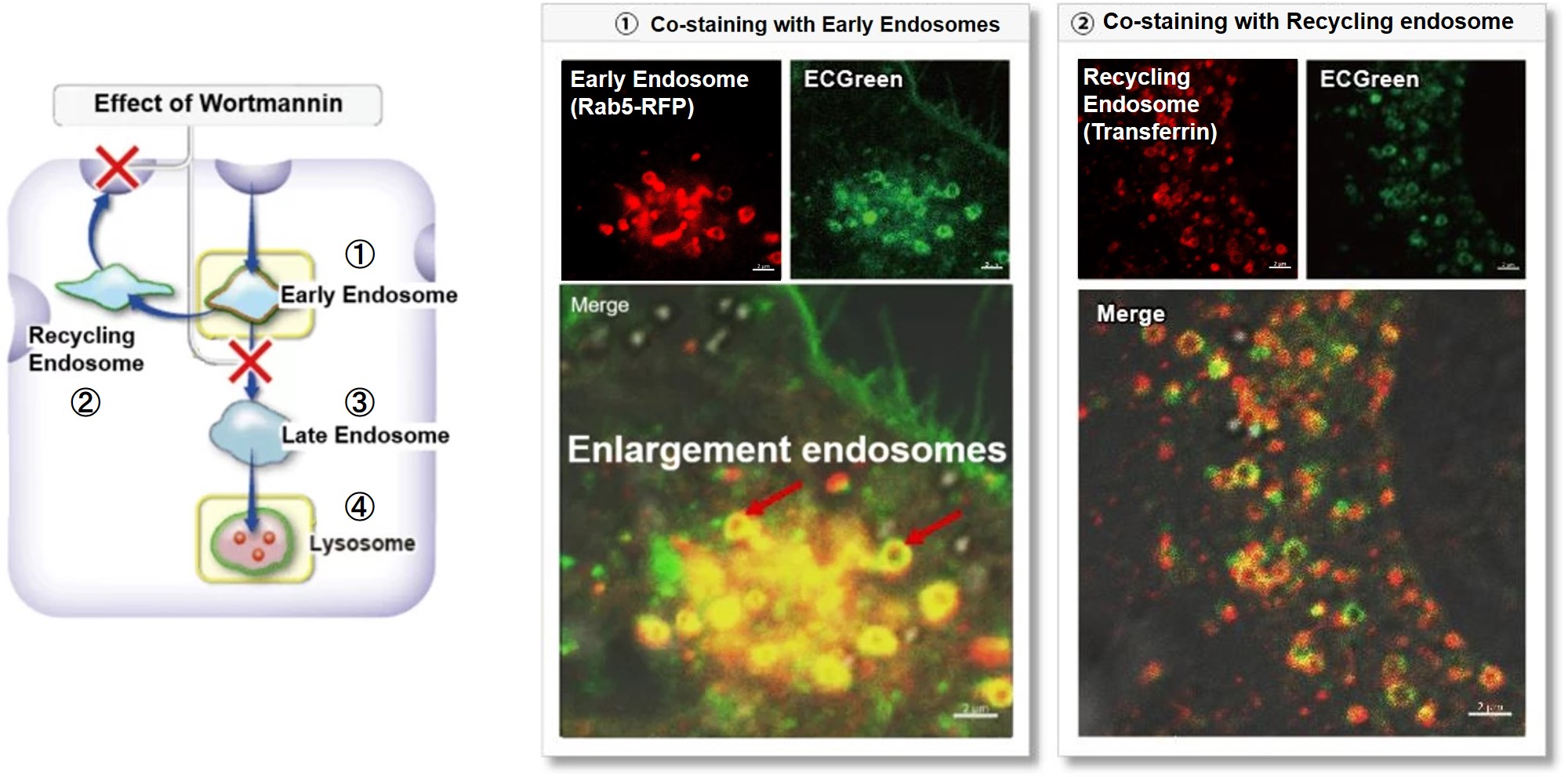
Endosomes (ECGreen, green): Ex. 405 nm / Em. 500 – 560 nm
Early endosomes (Rab5-RFP, red): Ex. 561 nm / Em. 560 – 620 nm
Recycling endosome (Transferrin-Alexa fluor 488 conjugate, red: pseudo-color): Ex. 488 nm / Em. 500 – 550 nm
Late endosomes (Rab7-RFP, red): Ex. 561 nm / Em. 560 – 620 nm
Lysosomes (Lamp1-RFP, red): Ex. 561 nm / Em. 560 – 620 nm
(1) Prepare HeLa cells in 8 wells of μ-Slide and incubate overnight.
(2) After washing with HBSS, 200 µl of Wortmannin (final concentration: 100 nmol/l) prepared in 10% FBS-containing MEM medium was added.
(3) Incubate at 37°C for 30 minutes
(4) 200 µl of ECGreen (diluted 1,000-fold) prepared in 10% FBS-containing MEM medium without removing the supernatant
(5) Incubate at 37°C for 30 minutes
(6) Wash the cells twice with HBSS and add MEM medium containing 10% FBS.
(7) Observation with a confocal laser microscope
Experimental example: Time-lapse imaging of Endosome localization
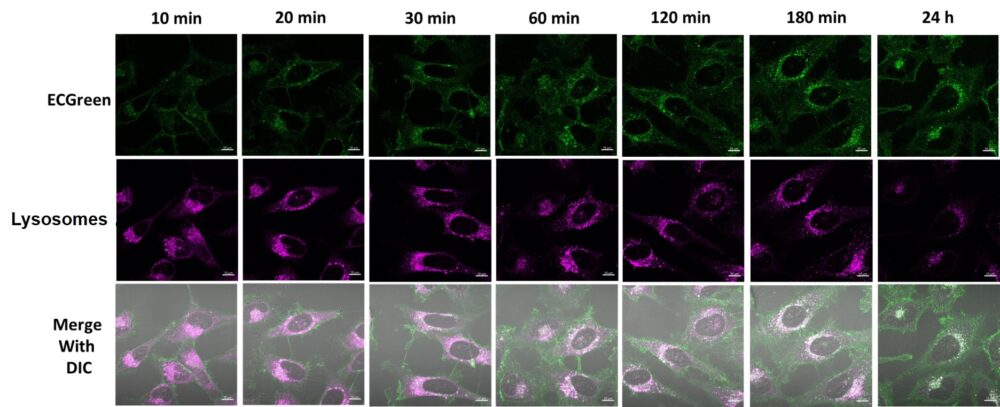
Endosomes (ECGreen, green): Ex. 405 nm / Em. 500 – 560 nm
Lysosome (Lysosome staining dye, red): Ex. 561 nm / Em. 600 – 700 nm
(1) HeLa cells and culture them overnight.
(2) Wash the cells once with HBSS.
(3) ECGreen (diluted 1,000-fold) prepared in 10% FBS-containing MEM medium and lysosomal staining dye (final concentration: 100 nmol/l) were added.
(4) Observe unwashed cells at each hour using a confocal laser microscope.
Visualization of EVs uptake via endocytic pathway
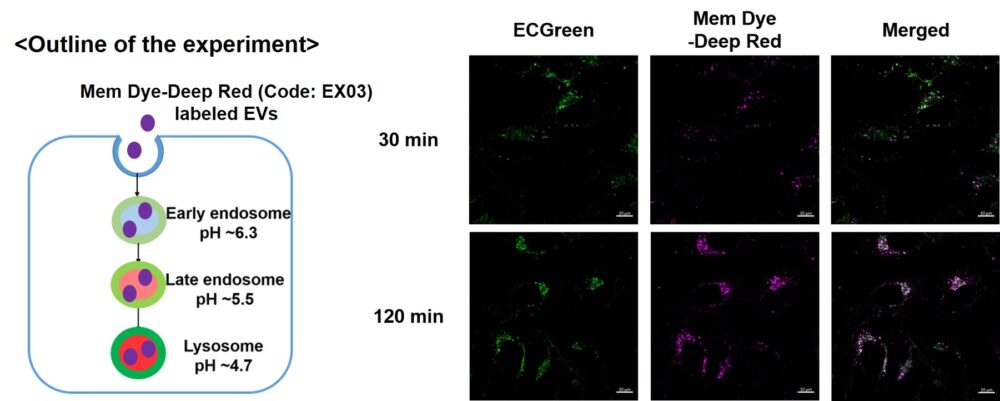
Mem Dye-labeled EVs are internalized via endocytosis:
HeLa cells were incubated with 10 μmol/L ECGreen for 30 min. Then, Mem Dye-Deep Red (Code: EX03) labeled EVs (quantified as 10 µg of protein) were added to HeLa cells. After 30 or 120 min incubation, the cells were washed and observed under a fluorescence microscope (Scale Bar: 10 µm).
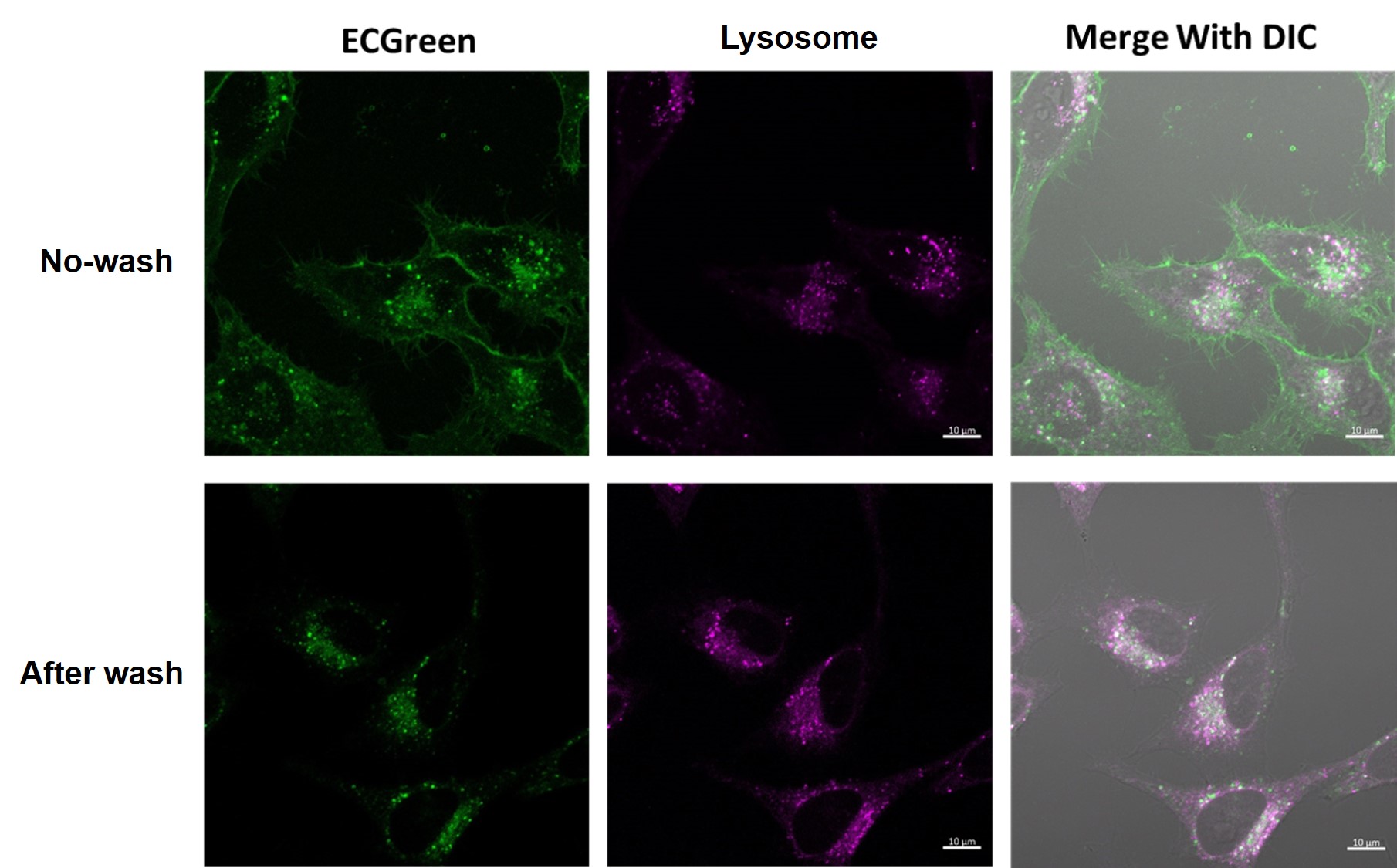
With or without washing after staining
When observation is performed without washing after staining, the image obtained has the following characteristics
No washing after staining:
Since the dye can remain in the cell membrane, endocytosis can be observed as it changes over time.
In this case, fluorescent signals are observed on the cell membrane because an excessive amount of dye is retained in the membrane.
With washing after staining:
Residual dye in excessive amounts on the cell membrane is removed, and fluorescent signals originating from endocytosis can be observed more clearly.
Experimental example: Observation of temperature-dependent endocytosis changes using floating cells
Temperature-dependent changes in the endocytosis of Jurkat cells were visualized using ECGreen-Endocytosis Detection and PlasMem Bright Red (product code: P505).
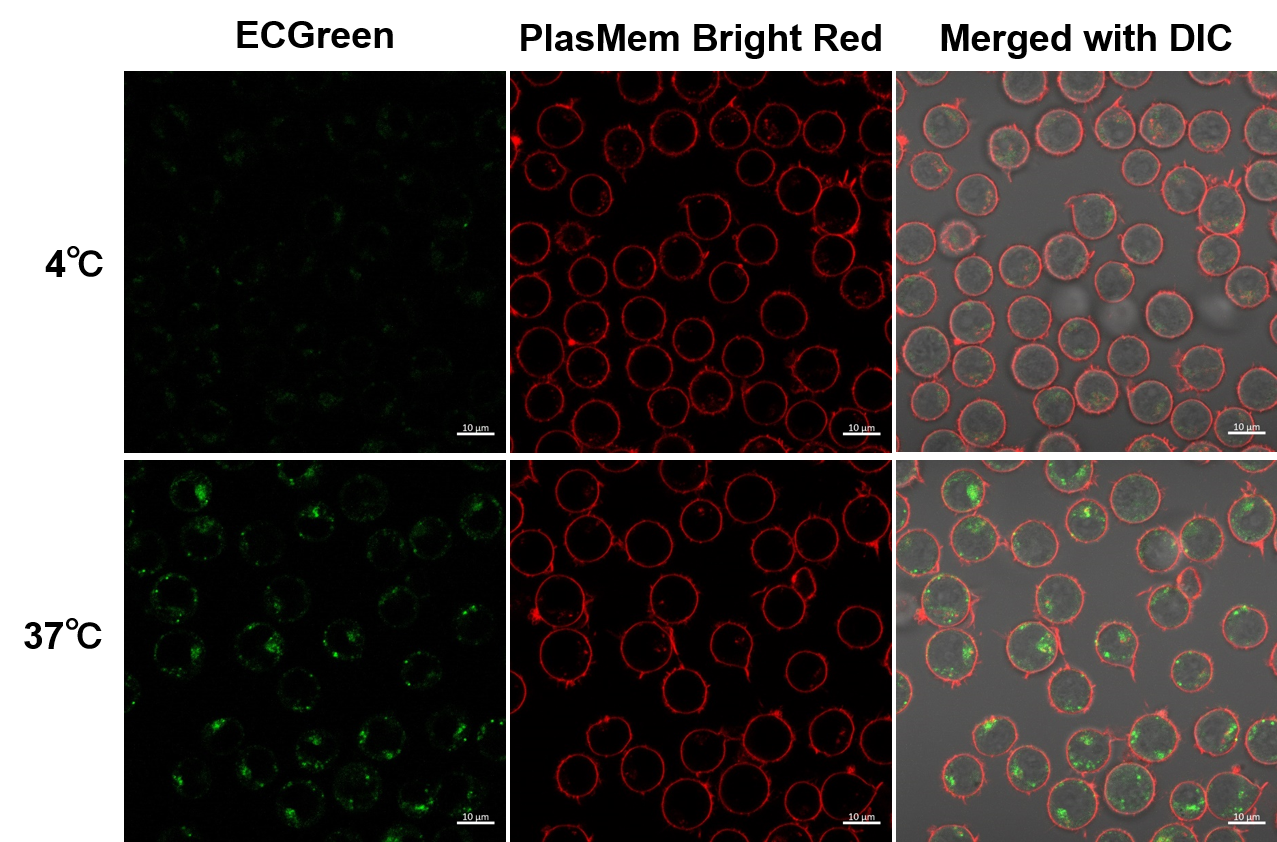
(Scale bar: 10 µm)
Endosomes (ECGreen, green): Ex. 405 nm / Em. 500 - 560 nm
Cell membrane (PlasMem Bright Red): Ex. 561 nm / Em. 560 - 700 nm
<Experimental procedure>
(1) Place Jurkat cell suspension (10% FBS, RPMI) in a sample tube and incubate at 4 °C or 37 °C for 30 minutes.
(2) Dilute the ECGreen solution 1000-fold using the suspension in (1).
(3) Incubate at 4 °C or 37 °C for 30 min.
(4) Wash cells twice with HBSS
(5) Add medium containing PlasMem Bright Red (100x dilution) and suspend cells.
(6) Transfer the suspension to an imaging plate and observe cells using a confocal laser microscope
Experimental Example: Observation of Endocytosis Sequence
A431 cells were stained with ECGreen (2000x dilution) for 5 minutes, washed with PBS, and observed in time-lapse under a fluorescence microscope.
The fluorescence intensity of ECGreen tended to increase around 10 minutes after the start of observation.

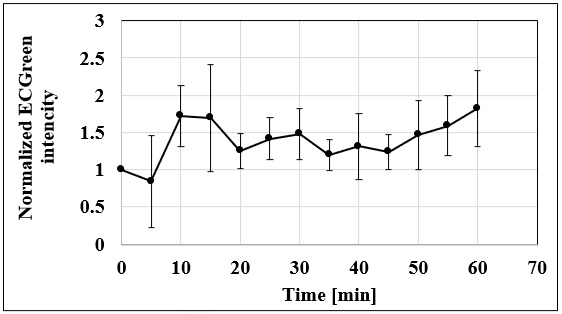
<Conditions of observation>
Fluorescence microscope: Olympus IX83 inverted research microscope
Filter: Semrock Excitation filter: FF01-387/11-25 Absorption filter: FF01-542/27
Imaging camera: QImaging (now TELEDYNE PHOTOMETRICS) EM-CCD camera Rolera EM-C2
Duration: 60 minutes, Interval: 5 minutes
This data was kindly provided by Dr. Yusuke Oba, Department of Cell Physiology, Graduate School of Medicine, Hokkaido University.
References
| No. | Sample | Instrument | Reference (Link) |
|---|---|---|---|
| 1) | Cell (HepG2) |
Fluorescent Microscope | Y. Miyakawa, M. Otsuka, K. Sekiba, K. Funato, K. Koike, "Humanized virus-suppressing factor inhibits hepatitis B virus infection by targeting viral cell entry ", Heliyon, 2021, doi:10.1016/j.heliyon.2021.e07586. |
| 2) | Cell (Human skin fibroblasts) |
Fluorescent Microscope | H. L. Marko, N. C. Hornig, R. C. Betz, P. Holterhus, J. Altmüller, H. Thiele, M. Fabiano, H. Schweikert, D. Braun, Ul Schiweizer, "Genomic variants reducing expression of two endocytic receptors in 46,XY differences of sex development", Hum. Mutat. 2022, doi:10.1002/humu.24325. |
| 3) | Cell (HeLa) |
Fluorescent Microscope | K. Qiu, R. Seino, G. Han, M. Ishiyama, Y. Ueno, Z. Tian, Y. Sun, J. Diao, "De Novo Design of A Membrane-Anchored Probe for Multidimensional Quantification of Endocytic Dynamics", Adv. Healthcare Mater., 2022, doi:10.1002/adhm.202102185. |
| 4) |
Cell (A431) |
Fluorescent Microscope | A. O. Sato, Y. Fujioka, S. Kashiwagi, A. Yoshida, M. Fujioka, H. Sasajima, A. Nanbo, M. Amano, Y. Ohba, "Interaction between PI3K and the VDAC2 channel tethers Ras-PI3K-positive endosomes to mitochondria and promotes endosome maturation", Cell Reports, 2023, doi:10.1016/j.celrep.2023.112229 |
Q & A
-
Q
Can I observe with a fluorescence microscope?
-
A
We recommend using a confocal microscope, but observation is also possible with a fluorescence microscope.
We have experience with DAPI filter set (Ex : 360/40 nm, Em : 460/50 nm).
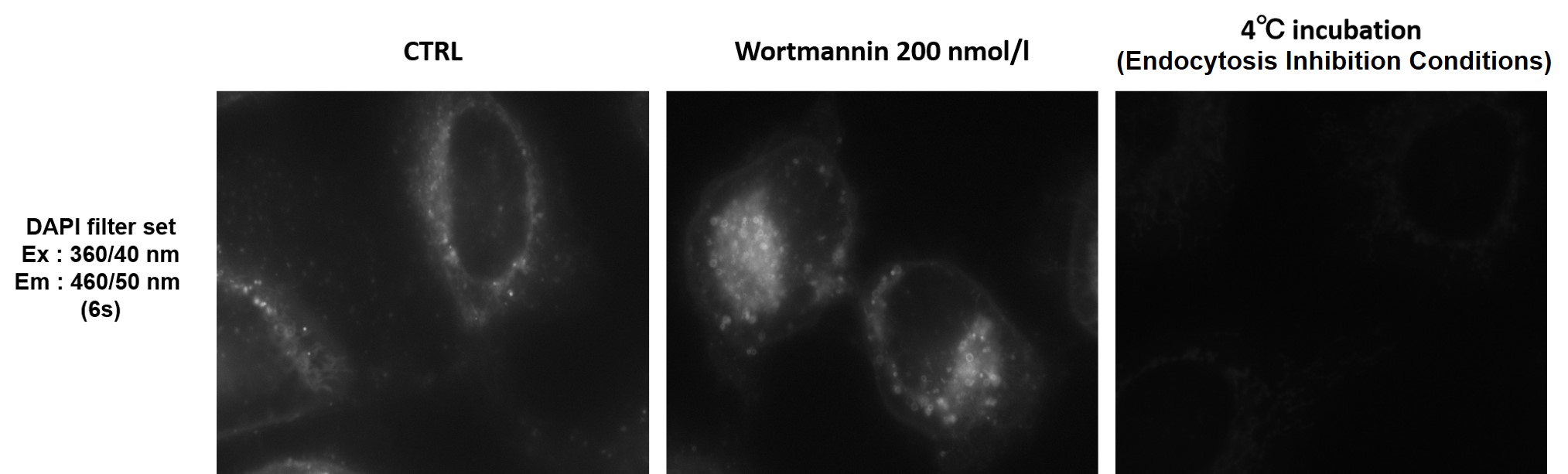
-
Q
Can I stain with a serum-containing medium?
-
A
Staining with a serum-containing medium is also possible.
We have experience in staining HeLa cells for up to 24 hours without cytotoxicity using a working solution prepared with a serum-containing medium.
Handling and storage condition
| Appearance: | Yellowish green liquid |
|---|---|
| Dye content: | To pass test |
| -20°C | |
|
Danger / harmful symbol mark |

|
|---|---|