|
Ferroptosis is a form of programmed cell death characterized by the accumulation of lipid peroxides to lethal levels and is distinct from other forms of cell death such as apoptosis, necroptosis, and autophagy. In the context of cancer, ferroptosis may act as a tumor suppressor mechanism, as cancer cells often have an increased susceptibility to ferroptosis due to their altered metabolism and increased levels of reactive oxygen species (ROS). Therapeutically, inducing ferroptosis in cancer cells has emerged as a promising strategy for cancer treatment, particularly for tumors that are resistant to traditional therapies such as chemotherapy and radiation. In addition, understanding the specific vulnerabilities of cancer cells to ferroptosis may aid in the design of targeted therapies that exploit these weaknesses, providing a potential avenue to overcome drug resistance and improve patient outcomes.
|
-
Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism
Click here for the original article: Pavan S. Upadhyayula, et. al., Nature Communications, 2023.
Point of Interest
- Cysteine and methionine deprivation (CMD) can synergize with the GPX4 inhibitor RSL3 to increase ferroptotic cell death and lipid peroxidation.
- A cysteine-depleted, methionine-restricted diet can improve the therapeutic response to RSL3 and prolong survival in a syngeneic orthotopic murine glioma model.
- This CMD diet profoundly alters in vivo metabolome, proteome, and lipidome.
-
Lysosomal cystine governs ferroptosis sensitivity in cancer via cysteine stress response
Click here for the original article: Robert V. Swanda et. al., Molecular Cell, 2023.
Point of Interest
- Depletion of cysteine induces adaptive ATF4 expression at the transcriptional level.
- A shortage of cystine in lysosomes stimulates ATF4 expression through the AhR signaling pathway.
- A weakened cysteine stress response increases sensitivity to ferroptosis during cysteine deprivation.
- CysRx promotes cancer cell ferroptosis through intracellular nutrient reprogramming.
-
Mitochondria regulate intracellular coenzyme Q transport and ferroptotic resistance via STARD7
Click here for the original article:Soni Deshwal et. al., Nature Cell Biology, 2023.
Point of Interest
- The rhomboid protease PARL cleaves STARD7, allowing its dual localization to the mitochondrial intermembrane space and cytosol.
- The mitochondrial STARD7 supports coenzyme Q synthesis, promotes oxidative phosphorylation, and maintains cristae morphology.
- Its cytosolic counterpart facilitates the transport of coenzyme Q to the plasma membrane and protects against ferroptosis.
- Overexpression of cytosolic STARD7 increases the resistance of cells to ferroptosis and reduces the availability of coenzyme Q in the mitochondria.
|
|
Related Techniques
|
- Intracellular / mitochondrial ferrous ion (Fe2+) detection
- FerroOrange(intracellular), Mito-FerroGreen(mitochondrial)
|
- NAD(H) and NADP(H) redox couples assay
- NAD/NADH and NADP/NADPH Assay Kit
|
|
|
- Lipid Peroxidation Assay
- Lipid Peroxidation Probe -BDP 581/591 C11-
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Mitochondrial membrane potential detection
- JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit
|
- Oxygen consumption rate assay
- Extracellular OCR Plate Assay Kit
|
- Glutathione Quantification
- GSSG/GSH Quantification Kit
|
- Cystine Uptake detection
- Cystine Uptake Assay Kit
|
- MDA detection
- MDA Assay Kit
|
- Mitophagy or autophagy detection
- Mitophagy Detection Kit, Autophagic Flux Assay Kit
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- Glycolysis/Oxidative phosphorylation Assay
-
- Glycolysis/OXPHOS Assay Kit
-
|
- Apoptosis detection in multiple samples
-
- Annexin V Apoptosis Plate Assay Kit
-
|
|
Related Applications
|
The simultaneous detection of lysosomal function with intracellular Fe2+
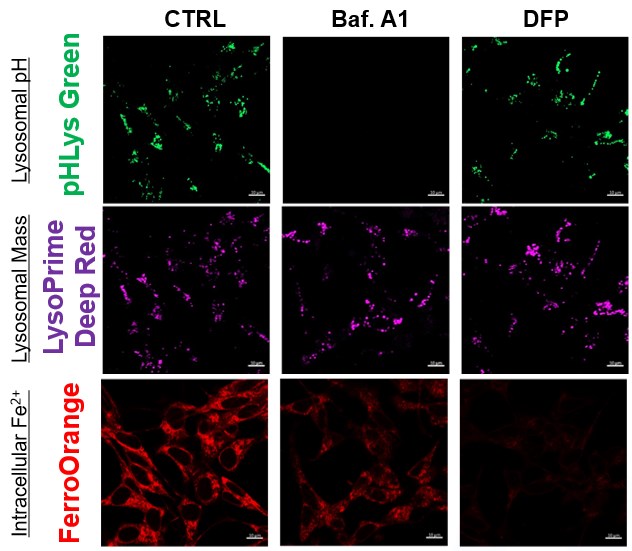
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis.
Reference: Ross A Weber, et. al., Mol Cell (2020)
Products in Use
- FerroOrange
- pHLys Green*
- LysoPrime Deep Red
*pHLys Green is available as the "Lysosomal Acidic pH Detection Kit-Green/Deep Red".
|
















